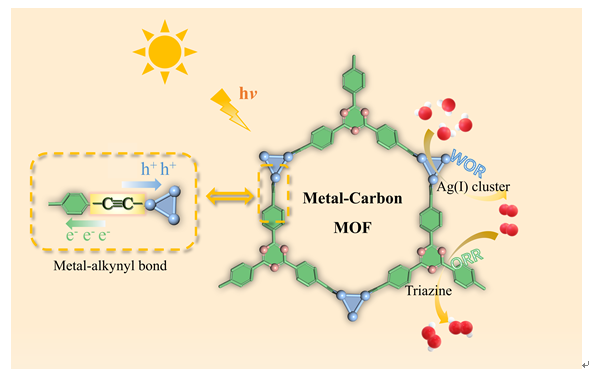
开发高效稳定的光催化剂用于太阳能驱动过氧化氢(H2O2)合成是当前绿色化学研究的重要方向. 基于金属-碳键(M-C)配位策略,我们成功设计并合成了一例新型银基金属有机框架(MOF)光催化剂Ag-TEPT(TEPT = 2,4,6-三(4-乙炔基苯基)-1,3,5-三嗪). 在模拟太阳光照下,该催化剂在不加任何牺牲剂的水-氧气体系中展现出优异的H2O2合成性能,产率可达2880 μmol g-1 h-1;即使在空气氛围中仍能维持2024 μmol g-1 h-1的高活性. 进一步的机理研究表明,H2O2的生成遵循两电子ORR与四电子WOR协同的双路径反应机制. 光致发光光谱和光电化学测试证实,催化剂中活性位点与银-碳配位键协同调控,不仅优化了催化剂的能带结构,还显著促进了光生电子-空穴对的有效分离,从而显著提升了光催化合成过氧化氢的活性.
陈慧滢
,
黄宁宇
,
廖培钦
. 基于银-碳键的新型金属有机框架用于高效光催化合成过氧化氢[J]. 化学学报, 0
: 20260106
-20260106
.
DOI: 10.6023/A25110383
The development of highly efficient and stable photocatalysts for solar-driven hydrogen peroxide (H2O2) production represents a pivotal direction in green chemistry research. Herein, we report the successful synthesis of a novel silver-based metal-organic framework (MOF) photocatalyst, Ag-TEPT (where TEPT = 2,4,6-tris(4-ethynylphenyl)-1,3,5-triazine), which was constructed via a metal-carbon (M-C) bond coordination strategy to bridge Ag(I) clusters with triazine-based linkers. Under simulated sunlight irradiation, Ag-TEPT exhibited an outstanding H2O2 production rate of 2880 μmol g-1 h-1 in a pure water-oxygen system without any sacrificial agents, a performance that significantly surpasses most reported photocatalysts. Impressively, it maintained a high production rate of 2024 μmol g-1 h-1 even in ambient air. Furthermore, Ag-TEPT demonstrated remarkable photocatalytic stability with negligible activity loss over three consecutive reaction cycles. Post-catalytic characterization confirmed its unvaried crystal structure, morphology, and surface chemical states, attesting to its potential as a high-performance and durable photocatalyst. Mechanistic studies revealed a dual-pathway reaction mechanism, wherein H2O2 generation proceeds via simultaneous 2e- ORR and 4e- WOR processes. Notably, the O2 produced from the 4e- WOR serves as an internal feedstock for the 2e- ORR, mitigating the dependency on exogenous O2 and thereby enhancing the overall catalytic efficiency. In addition, the band structure of AgC-MOF, constructed from Tauc plot fitting and Mott-Schottky measurements, provided thermodynamic validation that Ag-TEPT is suitable for the photocatalytic reduction of O2 to H2O2 and the oxidation of H2O to O2, but not for the direct oxidation of H2O to H2O2. Photoluminescence spectroscopy and photoelectrochemical measurements confirmed the exceptional photogenerated charge separation efficiency of AgC-MOF, a benefit derived from the superior electron transport capability of the Ag-alkynyl bonds, which led to markedly improved photocatalytic reaction kinetics. In particular, Ag-TEPT showed a substantially higher photocurrent density, attributable to the excellent photosensitivity of the triazine units within the TEPT linker.
[1] Yong Z.-J.;Ma, T.-Y. Angew. Chem. Int. Ed.2023, 62, e202308980.
[2] Shi X.-J.; Back S.; Gill T. M.; Siahrostami S.;Zheng X.-L. Chem.2021, 7, 38.
[3] Yi Y.-H.; Wang L.; Li G.;Guo, H.-C. Catal. Sci. Technol.2016, 6, 1593.
[4] Du M.-L.; Lu S.-L.; Dong W.-F.; Chen M.-Q.; Duan F.; Liu W.-H.;Yan, S.-R. Acta Chim. Sinica.2025, 83, 685.
[5] Ma D.; Xu M.; Ding L.; Guo J.-K.; Yang Y.; Dou W.; Li H.; Chen F.-J.;Tang Y.Angew. Chem. Int. Ed. 2025, doi: 10.1002/anie.202521029.
[6] Zuo Q.; Chu B.-X.; Ye X.-H.; Li F.-Y.; Li L.;Xu, Q. J. Am. Chem. Soc.2025, 147, 34681.
[7] Zeng X.-K.; Liu Y.; Hu X.-Y.;Zhang X.-W. Green Chem.2021, 23, 1466.
[8] Wu Q.-Y.; Zhang C.-X.; Sun K.;Jiang, H.-L. Acta Chim. Sinica.2020, 78, 688.
[9] Wang W.-J.; Chen D.; Li F.-Y.; Xiao X.;Xu Q. Chem.2024, 10, 86.
[10] Chen X.; Xia R. Q.; Deng Q. M.; Li Y. Q.; Chen M. H.; Li Y. G.; Cai X. C.; Titov A. A.; Filippov O. A.; Shubina E. S.; Wei R. J.; Ning G. H.;Li, D. Chin. J. Chem.2025, 43, 2277.
[11] Wang Y. X.; Zheng F.; Song D. X.; Niu B. Y.; Deng L. Q.;Zhang, X. M. Chin. J. Chem.2024, 42, 1093.
[12] Liu X.-G.; Shan Y.-Y.; Zhang S.-T.; Kong Q.-Q.;Pang, H. Green Energy Environ.2023, 8, 698.
[13] Shiraishi Y.; Kanazawa S.; Sugano Y.; Tsukamoto D.; Sakamoto H.; Ichikawa S.;Hirai T. ACS Catal.2014, 4, 774.
[14] Kondo Y.; Kuwahara Y.; Mori K.;Yamashita H. Chem.2022, 8, 2924.
[15] Moon G.; Kim W.; Bokare A. D.; Sung N.;Choi, W. Energy Environ. Sci.2014, 7, 4023.
[16] Sayed M.; Yu J.-G.; Liu G.;Jaroniec M. Chem. Rev.2022, 122, 10484.
[17] MATSUOKA M.; IINO K.; CHEN H.;ANPO, M. Res. Chem. Intermed.2004, 31, 153.
[18] Hui-Ying C.; Huang J. R.; Liu J. C.; Huang N. Y.; Chen X. M.;Liao, P. Q. Angew. Chem. Int. Ed.2024, 63, e202412553.
[19] Tang Y. Y.; Luo X.; Xia R. Q.; Luo J.; Peng S. K.; Liu Z. N.; Gao Q.; Xie M.; Wei R. J.; Ning G. H.;Li, D. Angew. Chem. Int. Ed.2024, 63, e202408186.
[20] Xia R. Q.; Liu Z. N.; Tang Y. Y.; Wu T.; Luo X.; Deng Q. M.; Ning G. H.;Li, D. Angew. Chem. Int. Ed.2025, 64, e202514091.
[21] Mohata S.; Majumder P.;Banerjee, R. Chem. Soc. Rev.2025, 54, 6062.
[22] Gong Z.-H.; Gao Y.; Li J.; Cai Z.-C.; Liu N.-F.;Jiang, J.-Z. Angew. Chem. Int. Ed.2025, 64, e202423205.
[23] Liu X.-Q.; Huang R.-R.; Peng L.-Y.; Yang J.-L.; Yan J.-B.; Zhai B.-B.; Luo Y.; Zhang C.; Tan S.-W.; Liu X.-Y.; Ding L.-P.;Fang, Y. Angew. Chem. Int. Ed.2024, 64, e202414472.
[24] Sun R.-X.; Yang X.-J.; Hu X.-L.; Guo Y.-T.; Zhang Y.-Q.; Shu C.; Yang X.; Gao H.; Wang X.-Y.; Hussain I.;Tan, B. Angew. Chem. Int. Ed.2024, 64, e202416350.
[25] Yang H.-Y.; Chen X.-K.; Mou Y.-J.; Li Q.; Liu J.-J.; Sun L.-J.; Zhai S.-L.; Deng W. Q.;Wu H. Small.2024, 20, 2406737.
[26] Chen H.-Y.; Zhao Z.-H.; Huang N.-Y.; Huang J.-R.;Liao, P.-Q. J. Am. Chem. Soc.2025, 147, 38484.
[27] Jiang L.; Lin L.; Wang Z.-H.; Ai H.-Y.; Jia J.-T.;Zhu, G.-S. J. Am. Chem. Soc.2024, 146, 22930.
[28] De Riggi N.; Imberdis A.; Nicolas E.;Cantat T. Organometallics.2024, 43, 2466.
[29] Sun Q.; Cai L.-L.; Ma H.-H.; Yuan C.-X.;Xu W. ACS Nano.2016, 10, 7023.
[30] Qin Y.-Y.; Wang Y.; Lu J.; Xu L.-L.;Wong, W. Y. Angew. Chem. Int. Ed.2024, 64, e202418269.
[31] Jiang L.; Jia J.-T.; Ma Y.-H.; Tian Y.-Y.; Zou X.-Q.;Zhu G.-S. Chem.2024, 10, 557.
[32] Liu J.; Chen Q.-W.; Cai K.; Li J.; Li Y.; Yang X.; Zhang Y.-J.; Wang Y.-F.; Tang H.; Zhao D.-H.;Wu K. Nat. Commun.2019, 10, 2545.
[33] Le´tinois-Halbes U.; Pale P.;Berger, S. J. Org. Chem.2005, 70, 9185.
[34] Feng Y.-L.; Wang G.-R.; Chang Y.; Cheng Y.; Sun B.-B.; Wang L.-M.; Chen C.-Y.;Zhang H.-Y. Nano Lett.2019, 19, 4478.
[35] Xu L.; Xiao Y.; Yu Z. X.; Yang Y.; Yan C.;Huang, J. Q. Angew. Chem. Int. Ed.2024, 63, e202406054.
[36] Wang C.; Wang S.-J.;Kong F.-G. Inorg. Chem.2021, 60, 5034.



