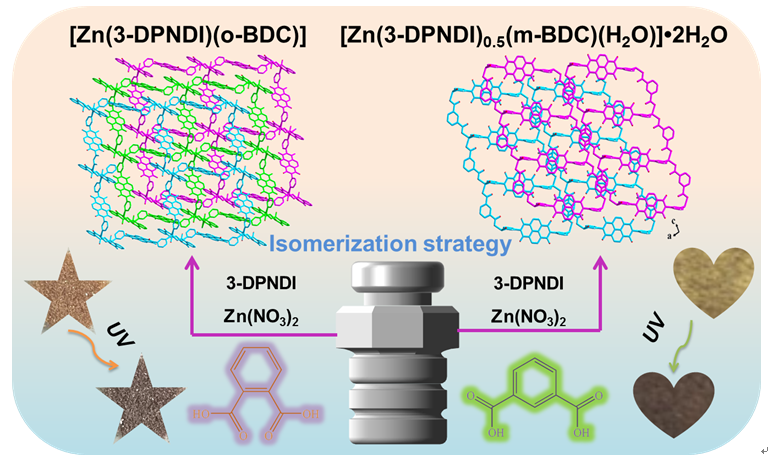
电子给-受体(ED-EA)的界面关系是决定光致变色配位聚合物(PCCPs)光致变色性能的关键因素,然而界面关系的调控仍处于初级阶段并且面临着严峻的挑战。本文将N, N-二-(3-吡啶基)-1,4,5,8-萘二酰亚胺(3-DPNDI)和Zn(NO3)2分别与苯二甲酸的两个同分异构体[邻苯二甲酸(o-BDC)和间苯二甲酸(m-BDC)]进行组装,构筑了两个PCCPs,[Zn(3-DPNDI)(o-BDC)] (1)和[Zn(3-DPNDI)0.5(m-BDC)(H2O)]•2H2O (2)。利用单晶X射线衍射(SCXRD)、粉末X射线衍射(PXRD)、傅里叶变换红外光谱(FT-IR)、热重(TG)、紫外-可见(UV-Vis)吸收光谱和电子顺磁共振(EPR)等技术对1和2进行了详细的表征。紫外光照射下,1和2呈现不同的光致变色性能,主要表现在颜色变化和光响应速率两个方面。尽管二者具有相同的变色时间,然而2吸光度的改变量Δabs远大于1,表明2具有更快的变色速率,这可以通过动力学曲线进一步证实。两个PCCPs光致变色性能的差异主要归因于同分异构电子给体的引入以及不同ED-EA间界面关系的形成。本文提出了一种通过引入同分异构电子给体调变CPs光致变色性能的策略,为构建具有可调的光响应功能材料奠定了坚实的基础。
The interfacial relationship between electron donors (ED) and acceptors (EA) is a key factor for determining the photochromic performance of photochromic coordination polymers (PCCPs). However, the regulation of the interfacial relationship is still in its infancy and continues to be a severe challenge. In this work, two PCCPs, [Zn(3-DPNDI)(o-BDC)] (1) and [Zn(3-DPNDI)0.5(m-BDC)(H2O)]•2H2O (2), have been successfully obtained through the assembly of N, N-di-(3-pyridyl)-1,4,5,8-naphthalenedicarboximide (3-DPNDI) and zinc nitrate (Zn(NO3)2) with two isomers of terephthalic acids [o-phthalic acid (o-BDC) and m-phthalic acid (m-BDC)]. Compound 1 and 2 have been characterized in detail by single-crystal X-ray diffraction (SCXRD), powder X-ray diffraction (PXRD), Fourier transform infrared spectroscopy (FT-IR), thermogravimetry (TG), ultraviolet-visible (UV-vis) absorption spectroscopy, and electron paramagnetic resonance (EPR). Upon ultraviolet light (~ 365 nm) irradiation, 1 and 2 exhibited different color change (from light brown to dark brown for 1 vs. from light brown to tan for 2) and photochromic response rates (color change time, 1 s for 1 vs. 1 s for 2; saturated time, 90 min for 1 vs. 30 min for 2). Although both 1 and 2 have the same color change time (1 s), the change of absorbance Δabs of 2 (0.07) is much larger than that of 1 (0.02), indicating that 2 has a faster photoresponse rate. Meanwhile, this result was further confirmed by the kinetic curves (1: 0.0078 s-1 and 2: 0.0110 s-1). The significantly different photochromic properties of the CPs are mainly attributed to the introduction of isomeric electron donors, which gives rise to different weak interactions between ED and EA, viz. intermolecular electron transfer (ET) channels, demonstrating the subtle modulation effect of isomeric electron donors on the interfacial relationship of ED-EA and photochromic properties. This paper proposes a strategy for modulating the photochromic performance of CPs by introducing isomeric electron donors, which lays a solid foundation for the construction of controllable photoresponsive functional materials.
[1] Yao H.; Cao W. Y.; Wang J. W.; Yang F. X.; Qin S. N.; Wei T. B.; Shi B. B.; Lin, Q. Chin. J. Chem.,2025, 43, 1487.
[2] Li N. Y.; Liu D.; Abrahams B. F.; Lang, J. P. Chem. Commun.,2018, 54, 5831.
[3] Han X. T.; Yi Z. H.; Gao Y. F.; Wang Z. P.; Su T.; Wu, J. B,; Liang, Z. Q.; Li, J. Y. Inorg. Chem.,2025, 64, 20415.
[4] Zhang Q. Q.; Wang Y.; Braunstein P.; Lang, J. P. Chem. Soc. Rev.,2024, 53, 5227.
[5] Hu F. L.; Wang H. F.; Guo D.; Zhang H.; Lang J. P.; Beves, J. E. Chem. Commun.,2016, 52, 7990.
[6] Li S. L.; Han M.; Li G. P.; Li M.; He G.; Zhang, X. M. J. Am. Chem. Soc.,2019, 141, 12663.
[7] Wang J. F.; Hao P. F.; Zhang, S. M. Xu, Y.; Qin J.; Shen J. J.; Fu, Y. L. Chem. Eng. J.,2025, 519, 165131.
[8] Yu B.; Xiu Y. Y.; Zhang S. M.; Wang, X. J. Am. Chem. Soc.2025, 147, 26626.
[9] Ovalle M.; Doellerer D.; Feringa, B. L. Angew. Chem. Int. Ed.,2025, 64, e202501872.
[10] Luo W. F.; Castán, J. M. A. C.; Mirani, D. Riquelme, A. J.; Sachan A. K.; Kurman O.; Kim S.; Faini F.; Zimmermann P.; Hinderhofer A.; Patel Y.; Frei A. T.; Moser J. E.; Ramirez D.; Schreiber F.; Maldivi P.; Seo J. Y.; Tress W.; Grancini G.; Demadrille R.; Milić J. V. Adv. Mater.,2025, 37, 2420143.
[11] Zhang S. M.; Hao P. F.; Yu W. Y.; Zhu H. H.; Yang H. Y.; Shen J. J.; Fu, Y. L. Acta Chim. Sin.,2025, 83, 1356.
(inChinese).
(张士民, 郝朋飞, 于炜玉, 朱慧慧, 杨海英, 沈俊菊, 付云龙. 化学学报, 2025, 83, 1356).
[12] Han S. D.; Hu J. X.; Wang, G. M. Coord. Chem. Rev.,2022, 452, 214304.
[13] Chen S. L.; Yang Q. T.; Shen X.; Cheng F. X.; Liu, J. J. Inorg. Chem.,2025, 64, 3008.
[14] Zhao G. Z.; Jia J. F.; Liu, J. J. Cryst. Growth Des.,2025, 25, 9507.
[15] Lang F. F.; Pang J. D.; Bu X. H. eScience,2024, 4, 100231
[16] Xue J. H.; Yang D. D.; Shi Y. S.; Yang Y. Y.; Ma Q.; Zheng, X. J. Inorg. Chem. Front.,2025, 12, 8315
[17] Di Y. M.; Song Y. P.; Zhang S. Q.; Lin, M. J. Inorg. Chem.,2025, 64, 6183.
[18] Zhang S. M.; Liu X. X.; Hao P. F.; Li G. P.; Shen J. J.; Fu, Y. L. Inorg. Chem.,2023, 62, 14912.
[19] Zhou B.; Cao L. H.; Huang M. F.; Yang Y.; Qi S. M.; Cao X. J.; Chen, X. Y. Angew. Chem. Int. Ed.,2025, 64, e202504645.
[20] Yu T. L.; Hao P. F.; Shen J. J.; Li H. H.; Fu, Y. L. Dalton Trans.,2016, 45, 16505.
[21] Sun C.; Wang M. S.; Li P. X.; Guo, G. C. Angew. Chem. Int. Ed.,2017, 56, 554.
[22] Gong T.; Yang X.; Fang J. J.; Sui Q.; Xi F. G.; Gao, E. Q. ACS Appl. Mater. Interfaces,2017, 9, 5503.
[23] Zhang J.; Yao Z. G.; Liao S. J.; Dai J. C.; Fu, Z. Y. J. Mater. Chem. A.,2013, 1, 4945.
[24] Liu J. J.; Xia S. B.; Liu D.; Hou J. Y.; Suo H. B.; Cheng, F. X. Dyes Pigm.,2020, 177, 108269.
[25] Hao P. F.; Gao B. H.; Li G. P.; Shen J. J.; Fu, Y. L. Inorg. Chem. Front.,2022, 9, 2852.
[26] Zhao W. N.; Yu G. J.; Xu L. P.; Han, L. Inorg. Chem. Comm.,2013, 34, 47.
[27] Zhang S. M.; Hao P. F.; Zhang Y. F.; Li G. P.; Shen J. J.; Yang H. Y.; Fu, Y. L. Inorg. Chem. Front.,2025, 12, 3919.
[28] Zhang S. M.; Hao P. F.; Yang H. Y.; Shen J. J.; Fu, Y. L. Inorg. Chem.,2025, 64, 12226.
[29] Wang Y.; Zhang Q. Q.; Huang X. Y.; Liu Q.; Lang, J. P. J. Am. Chem. Soc.,2025, 147, 22192.
[30] Shen Q. M.S. Thesis, Shanxi Normal University, Taiyuan, 2023 (in Chinese).
(申秋, 硕士论文, 山西师范大学, 太原, 2023.)
[31] Zhu H. H.M.S. Thesis, Shanxi Normal University, Taiyuan, 2021 (in Chinese).
(朱慧慧, 硕士论文, 山西师范大学, 临汾, 2021.)



