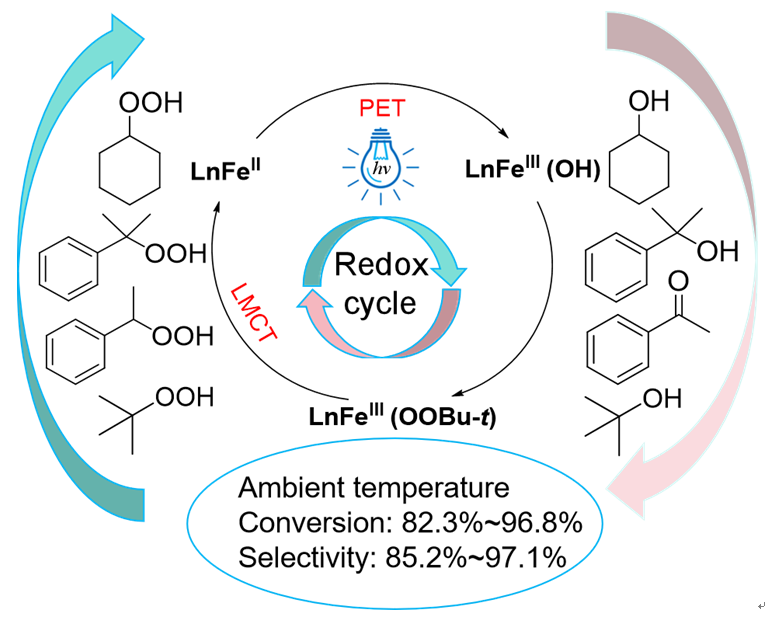
烷基过氧化氢(ROOH)是烃类选择氧化的初级产物,在温和条件下高效分解 ROOH 为高附加值醇、酮类含氧化学品是一个挑战性的课题。本研究以简单的铁盐为催化剂,在无碱条件下、以乙醇为溶剂、采用可见光照射,可高效催化分解多种 ROOH。金属盐的阴离子种类以及可见光波长等对催化活性有明显影响。常温下,采用 400 nm、3 W 的蓝光照射,ROOH 的转化率可以达到 82.3% ~ 96.8%,高附加值产物醇或酮的选择性达到 85.2% ~ 97.1%,催化机理的初步研究结果表明催化剂各组分间的相互作用对促进过氧化物的分解产生关键影响。
马逢宽
,
李雨茜
,
谢德重
,
张爽爽
,
陈晨
,
张巧红
. 常温可见光下铁盐催化分解烷基过氧化氢[J]. 化学学报, 0
: 25120405
-25120405
.
DOI: 10.6023/A25120405
Selective oxidation of C-H bond was regarded as the Holy Grail of chemistry. As one of the primary oxygenated products of selective oxidation of hydrocarbon, alkyl hydroperoxides (ROOHs) is necessary to be efficiently decomposed to the higher value-added oxygen-containing compounds such as alcohol and ketone. Usually, strong alkali of NaOH is used to decompose ROOHs to realize a higher selectivity to alcohol and ketone, which inevitably produces a large amount of alkali-containing waste potential destroying the environment. It is challenge to develop a green method to catalytically decompose ROOHs especially under the mild conditions. In the present work, at the absence of any alkali and under the irradiation of blue light, specific ferrous salt of FeCl2·4H2O was found to be able to act as an efficient catalyst to decompose various ROOHs in ethanol. Decomposition of ROOHs were all conducted in a quartz tubes (10 mL) under the photo irradiation of the LED lamp. Typically, TBHP aqueous solution (70 wt%, 0.8 mmol), metal salt (0.08 mmol), and solvent (1 mL) was added to the quartz tube. Then, the quartz tube was sealed with a sealing film and exposed to the LED lamp for 5 h under stirring. Modulating anionic and cationic species, wavelength of the light, and species of solvent showed obvious effect on the catalytic efficiency. At the ambient temperature under the photoirradiation with the blue light of 400 nm and 3 W, high conversion of 82.3%~96.8% could be obtained with the selectivity of 85.2%~97.1% to the oxygenated product of high value-added alcohol or ketone for a series of ROOHs. Characterization results of FT-IR and UV-Vis spectra indicated that the interaction happening between the ferrous ions, chloride ion, and the solvent molecules improved the visible light absorption ability and then played an important role to improve the catalytic activity under the blue light irradiation.
[1] Bryliakov, K. P. Acc. Chem. Res.1995, 28, 154-162.
[2] Zhu Z.; Zhang Q.; Xie D.; Liu H.; Wang H.; Shi L.; Chen, C. ACS Sustain. Chem. Eng.2022, 10, 13765-13774.
[3] Yuan J.; Liao X.; Wang H.; Yang G.; Tang, M. J. Phys. Chem. B.2009, 113, 1418-1422.
[4] Saint-Arroman R. P.; Didillon B.; de Mallmann A.; Basset J. M.; Lefebvre, F. Appl. Catal. A Gen.2008, 337, 78-85.
[5] Chaudhari R.; Mills, P. Chem. Eng. Sci.2004, 59, 5337-5344.
[6] Ramanathan A.; Hamdy M. S.; Parton R.; Maschmeyer T.; Jansen J. C.; Hanefeld, U. Appl. Catal. A Gen.2009, 355, 78-82.
[7] Gong Y.; Li M.; Li H.; Wang Y. Green Chem.2015, 17, 715-736.
[8] Shul'pin G.; Gradinaru J.; Kozlov, Y. Org. Biomol. Chem.2003, 1, 3611-3617.
[9] Zheng X.; Wang M.; Ma J.; Shi S.; Gao J.; Xu J.ACS Appl. Energy Mater. 2019, 2, 2176-2183.
[10] Qi L.; Qi X.; Wang L.; Feng L.; Lu S. Catal. Commun.2014, 49, 6-9.
[11] Zhou J.; Sun X.; Huang C.; Li J.; Chen, B. Appl. Catal. A Gen.2023, 656, 119116.
[12] Yu D.; Lin Y.; Zhou W.; Wang X.; Yu Z.; Hou, Y. Anpo M.; Yu J. C.; Zhang J.; Wang X. Langmuir.2025, 41, 4287-4295.
[13] Jin Y.; Zhi Q.; Wang H.; Zhan X.; Qi D.; Yu B.; Ding X.; Wang T.; Liu H.; Tang M.; Liu J.; Jiang, J. Natl. Sci. Rev.2025, 12, 396.
[14] Peng Q.; Peng Jia.; Cai Y.; Wang Z.; Yi R.; Shen C.; He, W. Acta Chimica. Sinica2025, 83, 1013-1017
(in Chinese). (彭琼慧,彭佳,蔡迎丽,王祖利,易荣楠,沈超,何卫民,化学学报,2025, 83, 1013-1017.)
[15] Nguyen X. S.; Pham T. D.; Ngo, K. D. Ceram. Int.2024, 50, 17957-17967.
[16] Maiti S. K. K.; Snavely K.; Ramanathan A.; Dakka J.; Nandi P.; Spry D. B. B.; Subramaniam, B. Ind. Eng. Chem. Res.2023, 62, 19238-19249.
[17] Tu H.; Wu Y.; Li Z.; Zhang P.; Wei C.; Yuan L.; Wang N.; Wang L.; Shi, W. Chem. Eng. J.2024, 485, 150089.
[18] De Laat J.; Le, T. App. Catal. B: Environ.2006, 66, 137-146.
[19] Yu T.; Li Q.; Zhao X.; Xia H.; Ma L.; Wang J.; Meng Y. S.; Shen, X. ACS Energy Lett.2017, 2, 2341-2348.
[20] Duan X.; Sha Q.; Li P.; Li T.; Yang G.; Liu W.; Yu E.; Zhou D.; Fang J.; Chen W.; Chen Y.; Zheng L.; Liao J.; Wang Z.; Li Y.; Yang H.; Zhang G.; Zhuang Z.; Hung S.-F.; Jing C.; Luo J.; Bai L.; Dong J.; Xiao H.; Liu W.; Kuang Y.; Liu B.; Sun X.Nat. Commun. 2024, 15, 1973.
[21] Zhou P.; Zhang J.; Xiong Z.; Liu Y.; Huo X.; Cheng X.; Li W.; Cheng F.; Zhang, Y. Appl. Catal. B Environ.2020, 265, 118264.
[22] Lotz H.; Gasperuzzo G.; Gómez-Sánchez, E. npj Mater. Degrad.2022, 6, 74.
[23] Wang Y.; Shi, X. Acta Chimica. Sinica2014, 72, 682-688
(in Chinese). (王媛,石晓燕,化学学报,2014, 72, 682-688.)
[24] Duh Y.-S.; Kuo H.-Y.; Kao, C.-S. J. Therm. Anal. Calorim.2017, 127, 1047-1059.
[25] Oh S.; Stache, E. E. ACS Catal.2023, 13, 10968-10975.
[26] Cui J.; Niu K.; Zhang R.; Liu H.; Yu S.; Xing L. Chem. Commun.2024, 60, 4310-4313.
[27] Tavadyan L.; Tonikyan H.; Sahakyan A.; Musaelyan, M. Int J Chem Kinet.2023, 55, 662-669.
[28] Ahn Y.; Roma G.; Colin, X. J. Phys. Chem. B.2024, 128, 12250-12258.
[29] Gao P.; Shen Z.; Chen Y.; Jiang T.; Ji Z.; Zhao G.; Yue J.; Hu Y.; Wang X.; Huang X.; Muhler M.; Yin L. J. Catal.2025, 442, 115900.
[30] Fredricks M. A.; Drees M.; Köhler K. ChemCatChem.2010, 2, 1467-1476.
[31] Bose S.; Pariyar A.; Biswas A. N.; Das P.; Bandyopadhyay, P. J. Mol. Catal. A Chem.2010, 332, 1-6.
[32] Biswas A. N.; Pariyar A.; Bose S.; Das P.; Bandyopadhyay P. Catal. Commun.2010, 11, 1008-1011.
[33] Msto R. K.; Othman H. O.; Al-Hashimi B. R.; Ali D. S.; Hassan D. H.; Hassan A. Q.; Smaoui, S. J. Food Qual.2023, 9, 5555608.


