Acta Chimica Sinica ›› 2021, Vol. 79 ›› Issue (8): 1073-1081.DOI: 10.6023/A21030083 Previous Articles Next Articles
Article
徐平a, 张西华a,*( ), 马恩a, 饶富a, 刘春伟b, 姚沛帆a, 孙峙b,*(
), 马恩a, 饶富a, 刘春伟b, 姚沛帆a, 孙峙b,*( ), 王景伟a
), 王景伟a
投稿日期:2021-03-07
发布日期:2021-07-19
通讯作者:
张西华, 孙峙
基金资助:
Ping Xua, Xihua Zhanga( ), En Maa, Fu Raoa, Chunwei Liub, Peifan Yaoa, Zhi Sunb(
), En Maa, Fu Raoa, Chunwei Liub, Peifan Yaoa, Zhi Sunb( ), Jingwei Wanga
), Jingwei Wanga
Received:2021-03-07
Published:2021-07-19
Contact:
Xihua Zhang, Zhi Sun
Supported by:Share
Ping Xu, Xihua Zhang, En Ma, Fu Rao, Chunwei Liu, Peifan Yao, Zhi Sun, Jingwei Wang. Selective Recovery of Lithium from Spent Lithium-ion Batteries Synergized by Carbon and Sulfur Elements[J]. Acta Chimica Sinica, 2021, 79(8): 1073-1081.
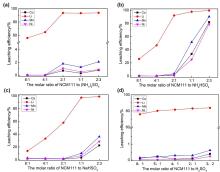
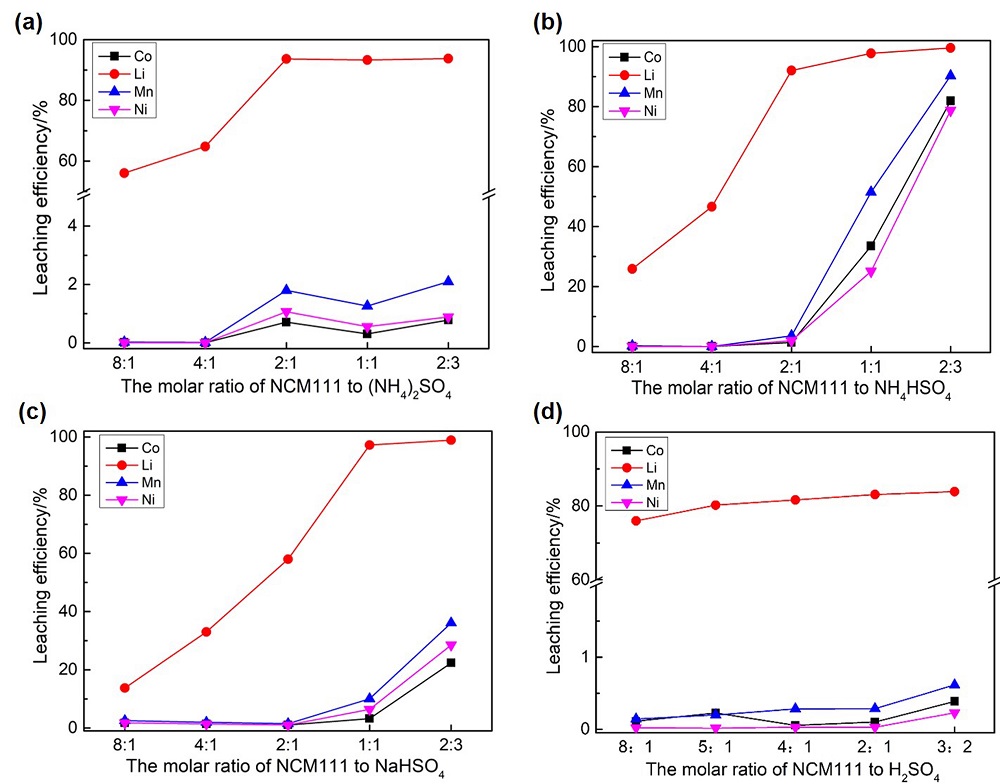
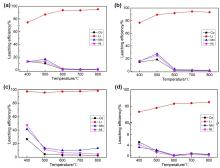
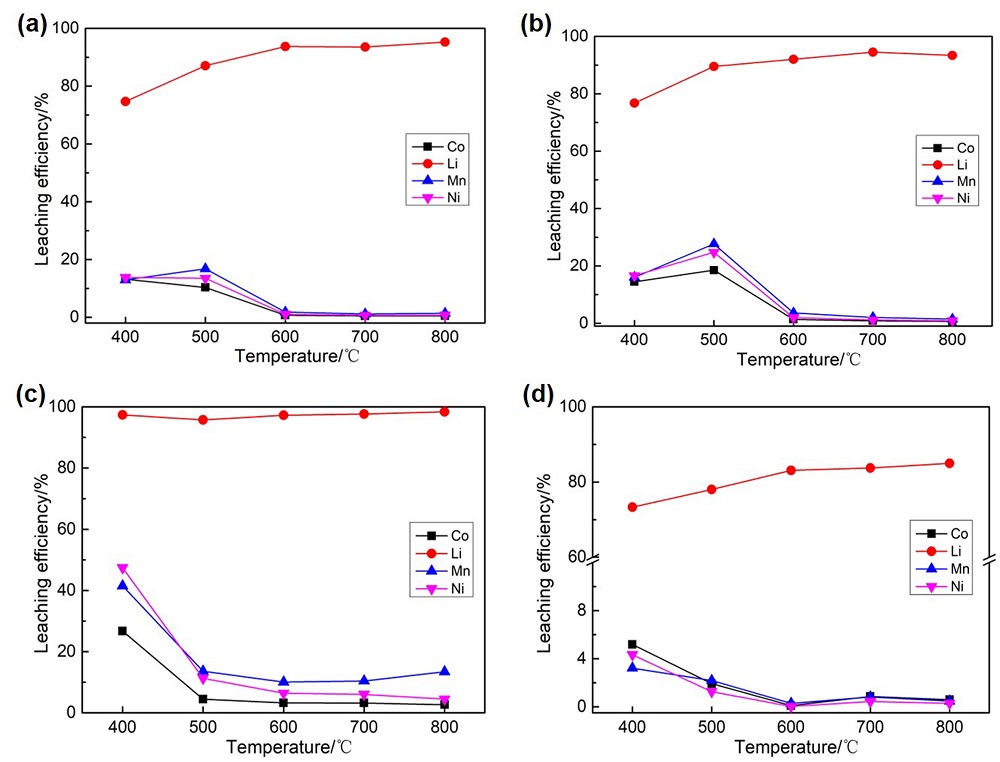
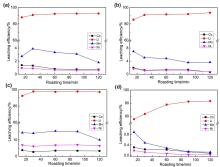
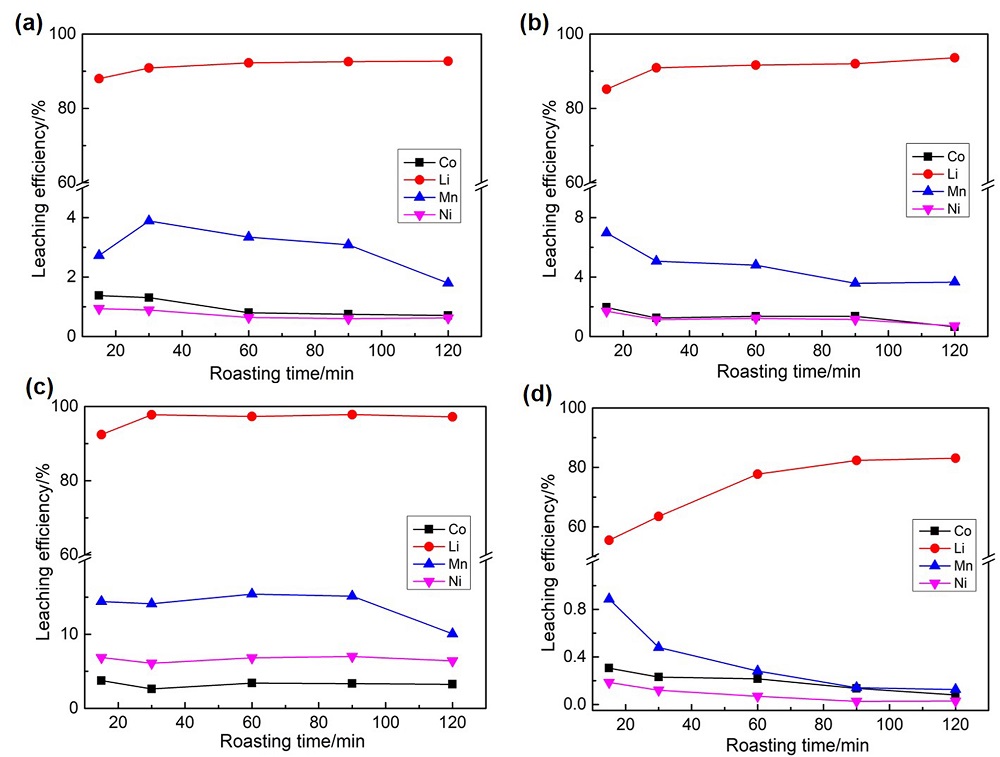
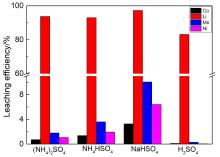
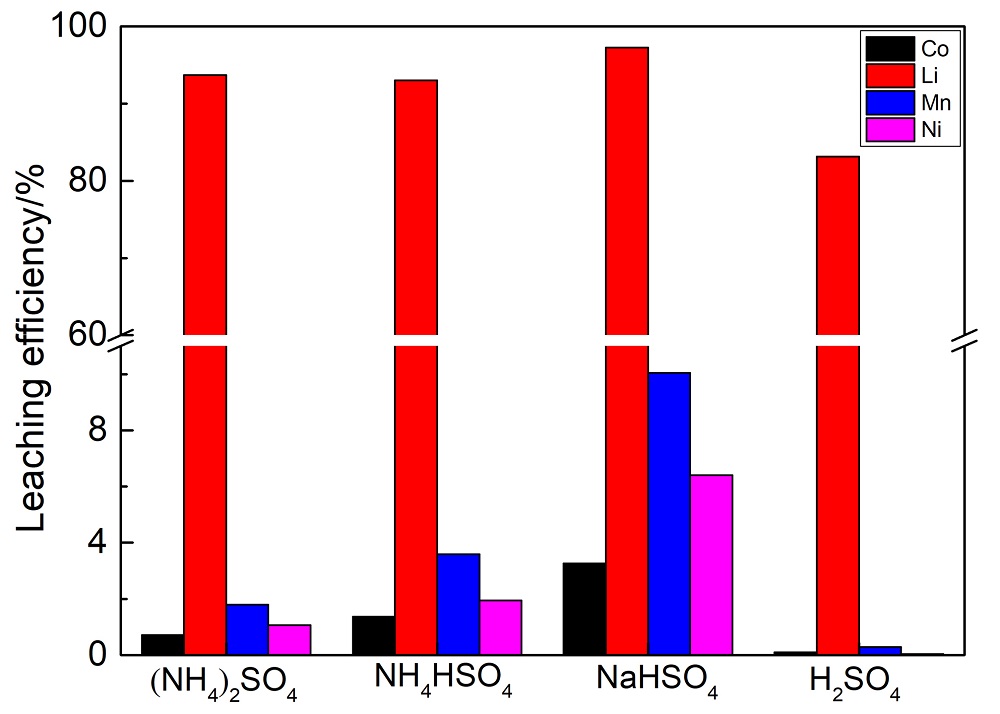

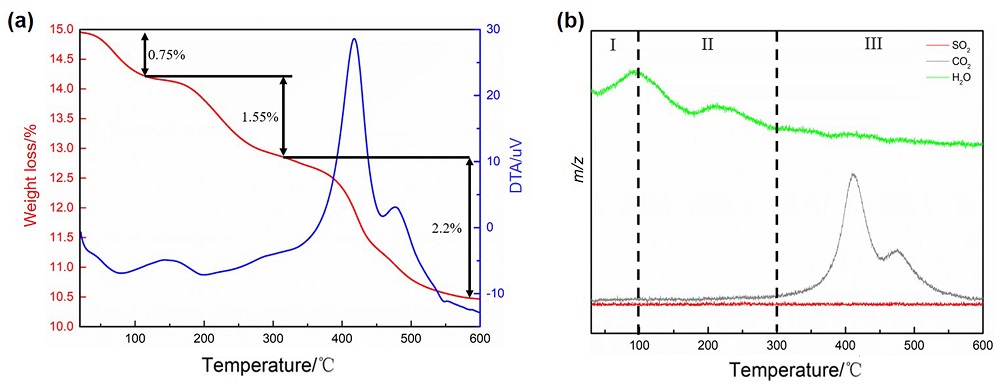
| [1] |
Zeng, X. L.; Li, J. H.; Singh, C. Crit. Rev. Environ. Sci. Technol. 2014, 44, 1129.
doi: 10.1080/10643389.2013.763578 |
| [2] |
Wang, M. M.; Zhang, C. C.; Zhang, F. S. Waste Manage. 2016, 51, 239.
doi: 10.1016/j.wasman.2016.03.006 |
| [3] |
Nie, H. H.; Xu, L.; Song, D. W.; Song, J. S.; Shi, X. X.; Wang, X. Q.; Zhang, L. Q.; Yuan, Z. H. Green Chem. 2015, 17, 1276.
doi: 10.1039/C4GC01951B |
| [4] |
Li, L.; Bian, Y. F.; Zhang, X. X.; Guan, Y. B.; Fan, E. S.; Wu, F.; Chen, R. J. Waste Manage. 2018, 71, 362.
doi: S0956-053X(17)30774-2 pmid: 29110940 |
| [5] |
Yang, Y.; Xu, S. M.; He, Y. H. Waste Manage. 2017, 64, 219.
doi: S0956-053X(17)30165-4 pmid: 28336333 |
| [6] |
Pagnanelli, F.; Moscardini, E.; Granata, G.; Cerbelli, S.; Agosta, L.; Fieramosca, A.; Toro, L. J. Ind. Eng. Chem. 2014, 20, 3201.
doi: 10.1016/j.jiec.2013.11.066 |
| [7] |
Mishra, D.; Kim, D. J.; Ralph, D.; Ahn, J. G.; Rhee, Y. H. Waste Manage. 2008, 28, 333.
doi: 10.1016/j.wasman.2007.01.010 |
| [8] |
Meshram, P.; Abhilash,
|
| [9] |
Kim, S.; Yang, D.; Rhee, K.; Sohn, J. Res. Chem. Intermed. 2014, 40, 2447.
doi: 10.1007/s11164-014-1653-2 |
| [10] |
Harper, G.; Sommerville, R.; Kendrick, E.; Driscoll, L.; Slater, P.; Stolkin, R.; Walton, A.; Christensen, P.; Heidrich, O.; Lambert, S.; Abbott, A.; Ryder, K.; Gaines, L.; Anderson, P. Nature 2019, 575, 75.
doi: 10.1038/s41586-019-1682-5 |
| [11] |
Georgi-Maschler, T.; Friedrich, B.; Weyhe, R.; Heegn, H.; Rutz, M. J. Power Sources 2012, 207, 173.
doi: 10.1016/j.jpowsour.2012.01.152 |
| [12] |
Dewulf, J.; Vorst, G. V.D.; Denturck, K.; Langenhove, H. V.; Ghyoot, W.; Tytgat, J.; Vandeputte, K. J.R. Resour. Conserv. Recycl. 2010, 54, 229.
doi: 10.1016/j.resconrec.2009.08.004 |
| [13] |
Ma, Y. Y.; Tang, J. J.; Wanaldi, R.; Zhou, X. Y.; Wang, H.; Zhou, C. Y.; Yang, J. J. Hazard Mater. 2020, 123491.
|
| [14] |
Li, H.; Xing, S. Z.; Liu, Y.; Li, F. J.; Guo, H.; Kuang, G. ACS Sustainable Chem. Eng. 2017, 5, 8017.
doi: 10.1021/acssuschemeng.7b01594 |
| [15] |
He, L. P.; Sun, S. Y.; Song, X. F.; Yu, J. G. Waste Manage. 2017, 64, 171.
doi: 10.1016/j.wasman.2017.02.011 |
| [16] |
Xiao, J. F.; Li, J.; Xu, Z. M.J Environ. Sci. Technol. 2017, 51, 11960.
doi: 10.1021/acs.est.7b02561 |
| [17] |
Hu, J. T.; Zhang, J. L.; Li, H. X.; Chen, Y. Q.; Wang, C. Y. J. Power Sources 2017, 351, 192.
doi: 10.1016/j.jpowsour.2017.03.093 |
| [18] |
Wang, D. H.; Zhang, X. D.; Chen, H. J.; Sun, J. Y. Miner. Eng. 2018, 126, 28.
doi: 10.1016/j.mineng.2018.06.023 |
| [19] |
Wang, D. H.; Wen, H.; Chen, H. J.; Yang, Y. J.; Liang, H. Y.J. Chem. Res. Chin. Univ. 2016, 32, 674.
doi: 10.1007/s40242-016-5490-2 |
| [20] |
Peng, C.; Liu, F. P.; Wang, Z. L.; Wilson, B. P.; Lundström, M. J. Power Sources 2019, 415, 179.
doi: 10.1016/j.jpowsour.2019.01.072 |
| [21] |
Sun, J. Y.M.S. Thesis, Lanzhou University of Technology, Lanzhou, 2015 (in Chinese.)
|
|
( 孙建勇, 硕士论文, 兰州理工大学, 兰州, 2015.)
|
|
| [22] |
Wen, H. M.S. Thesis, Lanzhou University of Technology, Lanzhou, 2016 (in Chinese.)
|
|
( 文豪, 硕士论文, 兰州理工大学, 兰州, 2016.)
|
|
| [23] |
Lin, J.; Liu, C. W.; Cao, H. B.; Chen, R. J.; Yang, Y. X.; Li, L.; Sun, Z. Green Chem. 2019, 21, 5904.
doi: 10.1039/C9GC01350D |
| [24] |
Lin, J.; Li, L.; Fan, E. S.; Liu, C. W.; Zhang, X. D.; Cao, H. B.; Sun, Z.; Chen, R. J. ACS Appl. Mater. Interfaces 2020, 12, 18482.
doi: 10.1021/acsami.0c00420 |
| [25] |
Yang, Y. X.; Meng, X. Q.; Cao, H. B.; Lin, X.; Liu, C. W.; Sun, Y.; Zhang, Y.; Sun, Z. Green Chem. 2018, 20, 3121.
doi: 10.1039/C7GC03376A |
| [1] | Xiaoyu Gu, Jin Li, Qian Sun, Chaoyang Wang. Microcalorimetry Analysis of Thermal Runaway Process in Lithium-ion Batteries [J]. Acta Chimica Sinica, 2024, 82(2): 146-151. |
| [2] | Zhixiang Yuan, Hao Zhang, Sijia Hu, Botao Zhang, Jianjun Zhang, Guanglei Cui. Research Progress of Ion-initiated in situ Generated Solid Polymer Electrolytes for High-safety Lithium Batteries★ [J]. Acta Chimica Sinica, 2023, 81(8): 1064-1080. |
| [3] | Ziqi Li, Liwei Liu, Chenghui Mao, Changkai Zhou, Minqi Xia, Zhen Shen, Yue Guo, Qiang Wu, Xizhang Wang, Lijun Yang, Zheng Hu. Cobalt-Substituted Polyoxometalates as Soluble Mediators to Boost the Lithium-Sulfur Battery Performance [J]. Acta Chimica Sinica, 2023, 81(6): 620-626. |
| [4] | Zhao Zhenxin, Yao Yikun, Chen Jiajun, Niu Rong, Wang Xiaomin. A High-entropy Phosphate Cathode Host towards High-stability Lithium-sulfur Batteries [J]. Acta Chimica Sinica, 2023, 81(5): 496-501. |
| [5] | Jia Yanggang, Chen Shijie, Shao Xia, Cheng Jie, Lin Na, Fang Daolai, Mao Aiqin, Li Canhua. Preparation and High-performance Lithium-ion Storage of Cobalt-free Perovskite High-entropy Oxide Anode Materials [J]. Acta Chimica Sinica, 2023, 81(5): 486-495. |
| [6] | Junliang Zhou, Zhenxin Zhao, Tingyi Wu, Xiaomin Wang. Efficient Catalytic Conversion of Polysulfides in Multifunctional FeP/Carbon Cloth Interlayer for High Capacity and Stability of Lithium-sulfur Batteries [J]. Acta Chimica Sinica, 2023, 81(4): 351-358. |
| [7] | Wanying Chang, Yingying Tan, Jingyi Wu, Yingjie Liu, Jinhai Cai, Chunyan Lai. Study on the Properties of Polyethylene Oxide Based Solid State Electrolyte Enhanced by Three-Dimensional Structured Li6.28La3Zr2Al0.24O12 [J]. Acta Chimica Sinica, 2023, 81(12): 1708-1715. |
| [8] | Yalan Zhang, Zhixiang Yuan, Hao Zhang, Jianjun Zhang, Guanglei Cui. Research Progress of High-energy-density Solid-state Lithium Ion Batteries Employing Ni-rich Ternary Cathodes [J]. Acta Chimica Sinica, 2023, 81(12): 1724-1738. |
| [9] | Guanhua Zhang, Zihan Yang, Yue Ma. Effect of Mixing Strategy on Electrochemical Performance of Oxide/Sulfide Solid Electrolyte [J]. Acta Chimica Sinica, 2023, 81(10): 1387-1393. |
| [10] | Shishuo Liang, Shusen Kang, Dong Yang, Jianhua Hu. Interficial Engineering of Lithium Metal Anode for Sulfide Solid State Batteries [J]. Acta Chimica Sinica, 2022, 80(9): 1264-1268. |
| [11] | Shuang Zhang, Chengfei Yang, Yubo Yang, Ningning Feng, Gang Yang. Electrochemical Behaviors of LixMO (x=0.79, 0.30, 0.08; M=Ni/Co/Mn) Recycled from Spent Li-ion Batteries as Cathodic Catalyst for Lithium-Oxygen Battery [J]. Acta Chimica Sinica, 2022, 80(9): 1269-1276. |
| [12] | Jiawei He, Liu Jiao, Xueyi Cheng, Guanghai Chen, Qiang Wu, Xizhang Wang, Lijun Yang, Zheng Hu. Structural Regulation of Metal Organic Framework-derived Hollow Carbon Nanocages and Their Lithium-Sulfur Battery Performance [J]. Acta Chimica Sinica, 2022, 80(7): 896-902. |
| [13] | Xingyu Ma, Hui Sun, Jiang Li, Zhiyang Liu, Hongjun Zhou. “Continuous” Nitrogen Reduction Synthesis of Ammonia Based on Li-N2 Battery System [J]. Acta Chimica Sinica, 2022, 80(7): 861-866. |
| [14] | Wenchao Bi, Linfeng Zhang, Jian Chen, Ruixue Tian, Hao Huang, Man Yao. Lithiation Mechanism and Performance of Monoclinic ZnP2 Anode Materials [J]. Acta Chimica Sinica, 2022, 80(6): 756-764. |
| [15] | Shouxiao Chen, Junke Liu, Weichen Zheng, Guozhen Wei, Yao Zhou, Juntao Li. Electron/ion Conductor Double-coated LiNi0.8Co0.1Mn0.1O2 Li-ion Battery Cathode Material and Its Electrochemical Performance [J]. Acta Chimica Sinica, 2022, 80(4): 485-493. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||