Acta Chimica Sinica ›› 2021, Vol. 79 ›› Issue (8): 1065-1072.DOI: 10.6023/A21040147 Previous Articles Next Articles
Article
投稿日期:2021-04-12
发布日期:2021-06-01
通讯作者:
王一波
基金资助:Received:2021-04-12
Published:2021-06-01
Contact:
Yibo Wang
Supported by:Share
Chengqiao Li, Yibo Wang. A Combination Method of Quantum Chemistry and Its Application to the Study of the Effects of Mercury on the Formation of Sulfuric Acid Aerosol[J]. Acta Chimica Sinica, 2021, 79(8): 1065-1072.
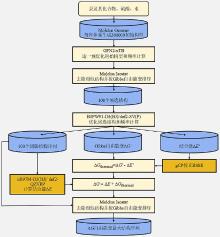
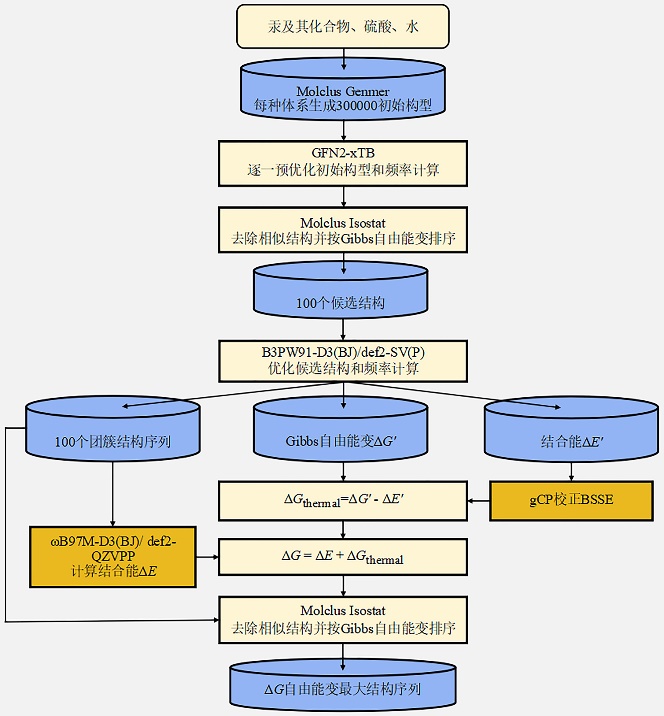
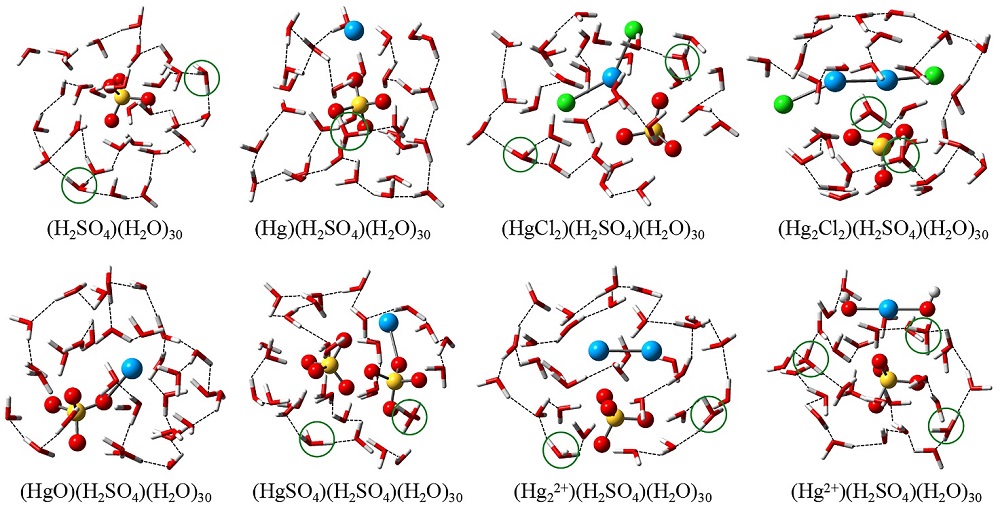
| No. | Reaction | ΔE | ΔH | ΔS | ΔG | ||||
|---|---|---|---|---|---|---|---|---|---|
| average | max | average | max | average | max | average | max | ||
| 1 | H2SO4+30H2O=>(H2SO4)(H2O)30 | –1391.81 | –1426.16 | –2367.10 | –2388.31 | –4.52 | –4.56 | –809.65 | –816.38 |
| 2 | Hg+H2SO4+30H2O=>(Hg)(H2SO4)(H2O)30 | –1417.62 | –1430.68 | –2397.68 | –2434.67 | –4.60 | –4.64 | –791.74 | –799.19 |
| 3 | Hg2Cl2+H2SO4+30H2O=>(Hg2Cl2)(H2SO4)(H2O)30 | –1429.97 | –1468.29 | –2512.99 | –2550.94 | –4.64 | –4.69 | –749.61 | –782.03 |
| 4 | HgSO4+H2SO4+30H2O=>(HgSO4)(H2SO4)(H2O)30 | –1885.06 | –1896.52 | –2857.13 | –2888.97 | –4.73 | –4.77 | –1291.35 | –1297.54 |
| 5 | HgO+H2SO4+30H2O=>(HgO)(H2SO4)(H2O)30 | –1802.43 | –1826.57 | –2787.05 | –2833.99 | –4.69 | –4.73 | –1177.92 | –1186.08 |
| 6 | HgCl2+H2SO4+30H2O=>(HgCl2)(H2SO4)(H2O)30 | –1411.97 | –1420.72 | –2461.15 | –2486.97 | –4.64 | –4.69 | –766.89 | –774.04 |
| 7 | Hg22++H2SO4+30H2O=>(Hg22+)(H2SO4)(H2O)30 | –2271.91 | –2295.89 | –3413.64 | –3456.49 | –4.69 | –4.73 | –1695.44 | –1700.54 |
| 8 | Hg2++H2SO4+30H2O=>(Hg2+)(H2SO4)(H2O)30 | –2715.04 | –2749.18 | –3818.61 | –3849.03 | –4.60 | –4.69 | –2051.08 | –2069.20 |
| No. | Reaction | ΔE | ΔH | ΔS | ΔG | ||||
|---|---|---|---|---|---|---|---|---|---|
| average | max | average | max | average | max | average | max | ||
| 1 | H2SO4+30H2O=>(H2SO4)(H2O)30 | –1391.81 | –1426.16 | –2367.10 | –2388.31 | –4.52 | –4.56 | –809.65 | –816.38 |
| 2 | Hg+H2SO4+30H2O=>(Hg)(H2SO4)(H2O)30 | –1417.62 | –1430.68 | –2397.68 | –2434.67 | –4.60 | –4.64 | –791.74 | –799.19 |
| 3 | Hg2Cl2+H2SO4+30H2O=>(Hg2Cl2)(H2SO4)(H2O)30 | –1429.97 | –1468.29 | –2512.99 | –2550.94 | –4.64 | –4.69 | –749.61 | –782.03 |
| 4 | HgSO4+H2SO4+30H2O=>(HgSO4)(H2SO4)(H2O)30 | –1885.06 | –1896.52 | –2857.13 | –2888.97 | –4.73 | –4.77 | –1291.35 | –1297.54 |
| 5 | HgO+H2SO4+30H2O=>(HgO)(H2SO4)(H2O)30 | –1802.43 | –1826.57 | –2787.05 | –2833.99 | –4.69 | –4.73 | –1177.92 | –1186.08 |
| 6 | HgCl2+H2SO4+30H2O=>(HgCl2)(H2SO4)(H2O)30 | –1411.97 | –1420.72 | –2461.15 | –2486.97 | –4.64 | –4.69 | –766.89 | –774.04 |
| 7 | Hg22++H2SO4+30H2O=>(Hg22+)(H2SO4)(H2O)30 | –2271.91 | –2295.89 | –3413.64 | –3456.49 | –4.69 | –4.73 | –1695.44 | –1700.54 |
| 8 | Hg2++H2SO4+30H2O=>(Hg2+)(H2SO4)(H2O)30 | –2715.04 | –2749.18 | –3818.61 | –3849.03 | –4.60 | –4.69 | –2051.08 | –2069.20 |
| [1] |
Sipilä, M.; Berndt, T.; Petäjä, T.; Brus, D.; Vanhanen, J.; Stratmann, F.; Patokoski, J.; Mauldin, R. L.; Hyvärinen, A. P.; Lihavainen, H.; Kulmala, M. Science 2010, 327, 1243.
doi: 10.1126/science.1180315 pmid: 20203046 |
| [2] |
Asaduzzaman, A.; Riccardi, D.; Afaneh, A. T.; Cooper, S. J.; Smith, J. C.; Wang, F.; Parks, J. M.; Schreckenbach, G. Acc. Chem. Res. 2019, 52, 379.
doi: 10.1021/acs.accounts.8b00454 |
| [3] |
Hong, Q.; Xie, Z.; Liu, C.; Wang, F.; Xie, P.; Kang, H.; Xu, J.; Wang, J.; Wu, F.; He, P.; Mou, F.; Fan, S.; Dong, Y.; Zhan, H.; Yu, X.; Chi, X.; Liu, J. Atmos. Chem. Phys. 2016, 16, 13807.
doi: 10.5194/acp-16-13807-2016 |
| [4] |
Li, L.; Zhang, Y. Y.; Jiao, C. Y.; Yao, Y. W.; Zhang, H.; Tian, Y. M. Environ. Monit. China 2019, 35, 40 (in Chinese.)
|
|
李亮, 张艳艳, 焦聪颖, 姚雅伟, 张辉, 田英明, 中国环境监测, 2019, 35, 40.)
|
|
| [5] |
Doyle, G. J. J. Chem. Phys. 1961, 35, 795.
doi: 10.1063/1.1701218 |
| [6] |
Jaecker, V. A.; Mirabel, P. Atmos. Environ. 1989, 23, 2053.
doi: 10.1016/0004-6981(89)90530-1 |
| [7] |
Sucarrat, M. T.; Francisco, J. S.; Anglada, J. M. J. Am. Chem. Soc. 2012, 134, 20632.
doi: 10.1021/ja307523b |
| [8] |
Kildgaard, J. V.; Mikkelsen, K. V.; Bilde, M.; Elm, J. J. Phys. Chem. A 2018, 122, 5026.
doi: 10.1021/acs.jpca.8b02758 pmid: 29741906 |
| [9] |
Humphries, R. S.; Schofield, R.; Keywood, M. D.; Ward, J.; Pierce, J. R.; Gionfriddo, C. M.; Tate, M. T.; Krabbenhoft, D. P.; Galbally, I. E.; Molloy, S. B.; Klekociuk, A. R.; Johnston, P. V.; Kreher, K.; Thomas, A. J.; Robinson, A. D.; Harris, N. R.P.; Johnson, R.; Wilson, S. R. Atmos. Chem. Phys. 2015, 15,13339.
|
| [10] |
Lu, T. Molclus program, Version 1.8.7, Beijing Kein Research Center for Natural Science, China, 2018, http://www.keinsci.com/research/molclus.html
|
| [11] |
Bannwarth, C.; Ehlert, S.; Grimme, S. J. Chem. Theory Comput. 2019, 15, 1652.
doi: 10.1021/acs.jctc.8b01176 |
| [12] |
Tsuzuki, S.; Honda, K.; Uchimaru, T.; Mikami, M.; Tanabe, K. J. Am. Chem. Soc. 2002, 124, 104.
pmid: 11772067 |
| [13] |
Hu, M.; Shang, D. J.; Guo, S.; Wu, Z. J. Acta Chim. Sinica 2016, 74, 385 (in Chinese.)
doi: 10.6023/A16020105 |
|
胡敏, 尚冬杰, 郭松, 吴志军, 化学学报, 2016, 74, 385.)
doi: 10.6023/A16020105 |
|
| [14] |
Elm, J.; Passananti, M.; Kurtén, T.; Vehkamäki, H. J. Phys. Chem. A 2017, 121, 6155.
doi: 10.1021/acs.jpca.7b05658 |
| [15] |
Yang, P.; Ye, Z. L.; Jiang, G. Y.; Li, Z.; Ding, C. F.; Hou, H. Q. Acta Chim. Sinica 2009, 67, 2031 (in Chinese.)
|
|
杨鹏, 叶招莲, 蒋公羽, 李周, 丁传凡, 侯惠奇, 化学学报, 2009, 67, 2031.)
|
|
| [16] |
Lu, Q.; Luo, Q.; Huang, S.; Li, Y.; Wan, J. J. Chem. Phys. A 2016, 120, 4560.
doi: 10.1021/acs.jpca.6b05529 |
| [17] |
Lu, Q.; Luo, Q.; Huang, S.; Li, Y. Phys. Chem. Chem. Phys. 2017, 19, 28434.
doi: 10.1039/C7CP05610A |
| [18] |
Rasmussen, F. R.; Besel, V.; Mikkelsen, K. V.; Bilde, M.; Elm, J. J. Phys. Chem. A 2020, 124, 5253.
doi: 10.1021/acs.jpca.0c02932 pmid: 32463668 |
| [19] |
Dohm, S.; Bursch, M.; Hansen, A.; Grimme, S. J. Chem. Theory Comput. 2020, 16, 2002.
doi: 10.1021/acs.jctc.9b01266 |
| [20] |
Myllys, N.; Olenius, T.; Kurtén, T.; Vehkamäki, H.; Riipinen, I.; Elm, J. J. Phys. Chem. A 2017, 121, 4812.
doi: 10.1021/acs.jpca.7b03981 pmid: 28585824 |
| [21] |
Becke, A. D. J. Chem. Phys. 1993, 98, 5648.
doi: 10.1063/1.464913 |
| [22] |
Perdew, J. P.; Wang, Y. Phys. Rev. 1992, 45, 13244.
doi: 10.1103/PhysRevB.45.13244 |
| [23] |
Amaro-Estrada, J. I.; Maron, L.; Ramirez-Solis, A. Phys. Chem. Chem. Phys. 2014, 16, 8455.
doi: 10.1039/c3cp55339f pmid: 24668012 |
| [24] |
Castro, L.; Dommergue, A.; Renard, A.; Ferrari, C.; Ramirez-Solis, A.; Maron, L. Phys. Chem. Chem. Phys. 2011, 13, 16772.
doi: 10.1039/c1cp22154j |
| [25] |
Yoo, S.; Apra, E.; Zeng, X. C.; Xantheas, S. S. J. Phys. Chem. Lett. 2010, 1, 3122.
doi: 10.1021/jz101245s |
| [26] |
Neese, F.; Hansen, A.; Liakos, D. G. J. Chem. Phys. 2009, 131,064103.
|
| [27] |
He, Y.; Wang, Y. B. Acta Phys.-Chim. Sin. 2017, 33, 1149 (in Chinese.)
doi: 10.3866/PKU.WHXB201703291 |
|
何禹, 王一波, 物理化学学报, 2017, 33, 1149).
|
|
| [28] |
Najibi, A.; Goerigk, L. J. Chem. Theory Comput. 2018, 14, 5725.
doi: 10.1021/acs.jctc.8b00842 pmid: 30299953 |
| [29] |
Mardirossian, N.; Pestana, L. R.; Womack, J. C.; Skylaris, C. K.; Head-Gordon, T.; Head-Gordon, M. J. Phys. Chem. Lett. 2017, 8, 35.
doi: 10.1021/acs.jpclett.6b02527 pmid: 27936759 |
| [30] |
Mardirossian, N.; Gordon, M. H. J. Chem. Phys. 2016, 144,214110.
|
| [31] |
Boys, S. F.; Bernardi, F. Mol. Phys. 1970, 19, 553.
doi: 10.1080/00268977000101561 |
| [32] |
Kruse, H.; Grimme, S. J. Chem. Phys. 2012, 136,154101.
|
| [33] |
Grimme, S. Growing String Method, xTB Documentation, 2019, https://xtb-docs.readthedocs.io/en/latest/gsm.html
|
| [34] |
Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. A.; Nakatsuji, H.; Caricato, M.; Li, X.; Hratchian, H. P.; Izmaylov, A. F.; Bloino, J.; Zheng, G.; Sonnenberg, J. L.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Montgomery Jr., J. A.; Peralta, J. E.; Ogliaro, F.; Bearpark, M.; Heyd, J. J.; Brothers, E.; Kudin, K. N.; Staroverov, V. N.; Keith, T.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A.; Burant, J. C.; Iyengar, S. S.; Tomasi, J.; Cossi, M.; Rega, N.; Millam, J. M.; Klene, M.; Knox, J. E.; Cross, J. B.; Bakken, V.; Adamo, C.; Jaramillo, J.; Gomperts, R.; Stratmann, R. E.; Yazyev, O.; Austin, A. J.; Cammi, R.; Pomelli, C.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Zakrzewski, V. G.; Voth, G. A.; Salvador, P.; Dannenberg, J. J.; Dapprich, S.; Daniels, A. D.; Farkas, O.; Foresman, J. B.; Ortiz, J. V.; Cioslowski, J.; Fox, D. J. Gaussian 16, Revision B. 01, Gaussian, Inc., Wallingford CT, 2016.
|
| [35] |
Neese, F., Software update: the ORCA program system, version 4.2 WIREs: Comput. Mol. Sci. 2018, 8,e1327.
|
| [36] |
Werner, H. J.; Knowles, P. J.; Knizia, G.; Manby, F. R.; Schütz, M.; Celani, P.; Györffy, W.; Kats, D.; Korona, T.; Lindh, R.; Mitrushenkov, A.; Rauhut, G.; Shamasundar, K. R.; Adler, T. B.; Amos, R. D.; Bennie, S. J.; Bernhardsson, A.; Berning, A.; Cooper, D. L.; Deegan, M. J.O.; Dobbyn, A. J.; Eckert, F.; Goll, E.; Hampel, C.; Hesselmann, A.; Hetzer, G.; Hrenar, T.; Jansen, G.; Köppl, C.; Lee, S. J.R.; Liu, Y.; Lloyd, A. W.; Ma, Q.; Mata, R. A.; May, A. J.; McNicholas, S. J.; Meyer, W.; Miller III, T. F.; Mura, M. E.; Nicklass, A.; O’Neill, D. P.; Palmieri, P.; Peng, D.; Pflüger, K.; Pitzer, R.; Reiher, M.; Shiozaki, T.; Stoll, H.; Stone, A. J.; Tarroni, R.; Thorsteinsson, T.; Wang, M.; Welborn, M. MOLPRO, version 2018, a package of ab initio programs, http://www.molpro.net
|
| [37] |
Yang, Z. Z.; Meng, X. F.; Zhao, D. X.; Gong, L. D. Acta Chim. Sinica 2009, 67, 2074 (in Chinese.)
|
|
杨忠志, 孟祥凤, 赵东霞, 宫利东 化学学报 2009, 67, 2074).
|
|
| [38] |
Temelso, B.; Morrell, T. E.; Shields, R. M.; Allodi, M. A.; Wood, E. K.; Kirschner, K. N.; Castonguay, T. C.; Archer, K. A.; Shields, G. C. J. Phys. Chem. A 2012, 116, 2209.
doi: 10.1021/jp2119026 |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
