Acta Chimica Sinica ›› 2022, Vol. 80 ›› Issue (8): 1066-1070.DOI: 10.6023/A22020078 Previous Articles Next Articles
Article
投稿日期:2022-02-20
发布日期:2022-09-01
通讯作者:
刘思敏
基金资助:
Shimin Zhu, Xin Huang, Xie Han, Simin Liu( )
)
Received:2022-02-20
Published:2022-09-01
Contact:
Simin Liu
Supported by:Share
Shimin Zhu, Xin Huang, Xie Han, Simin Liu. Recognition and Luminescence Properties of N^C^N Pt(II) Complexes with Macrocyclic Host Cucurbit[10]uril[J]. Acta Chimica Sinica, 2022, 80(8): 1066-1070.
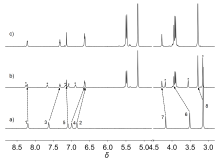
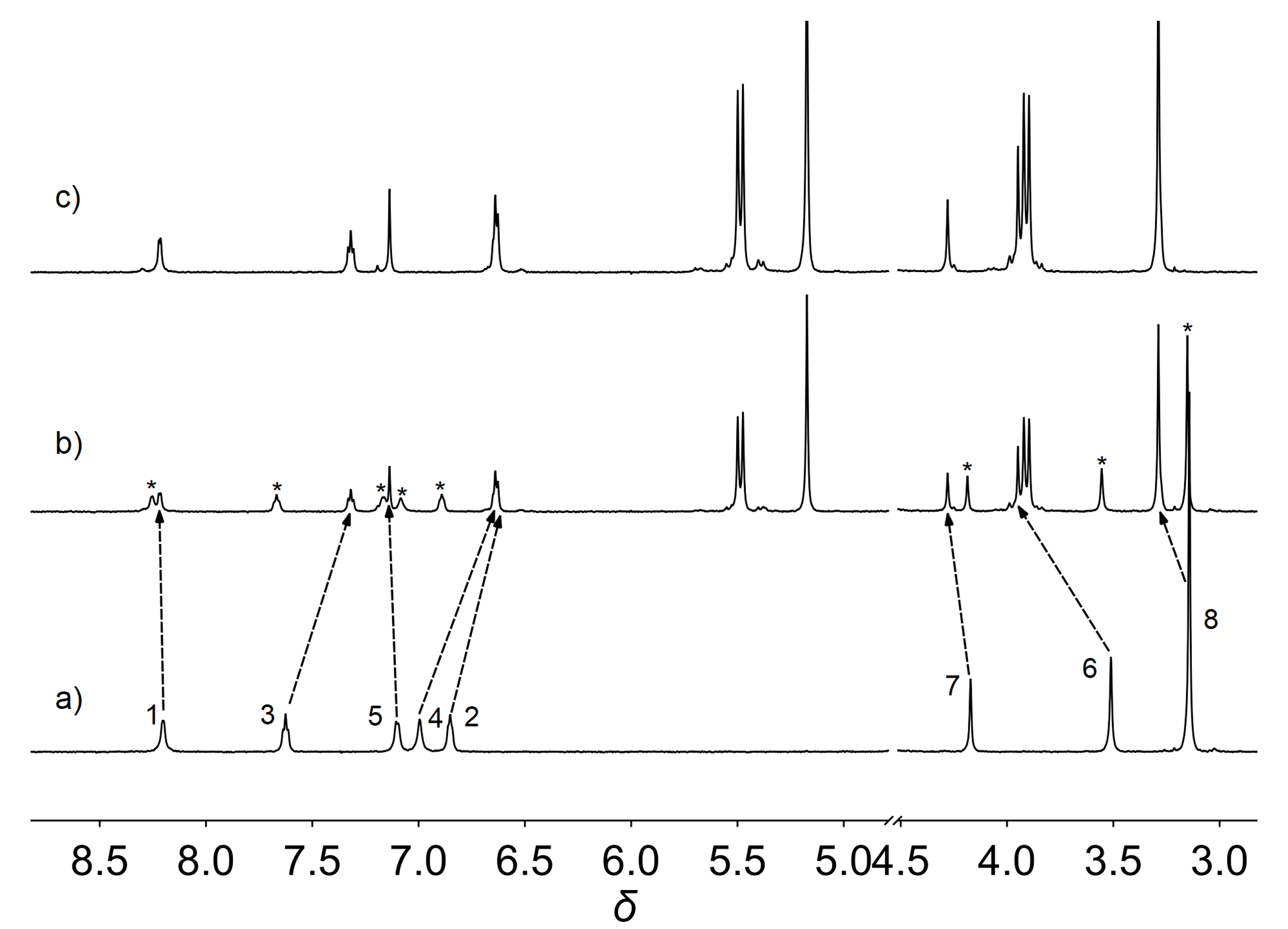
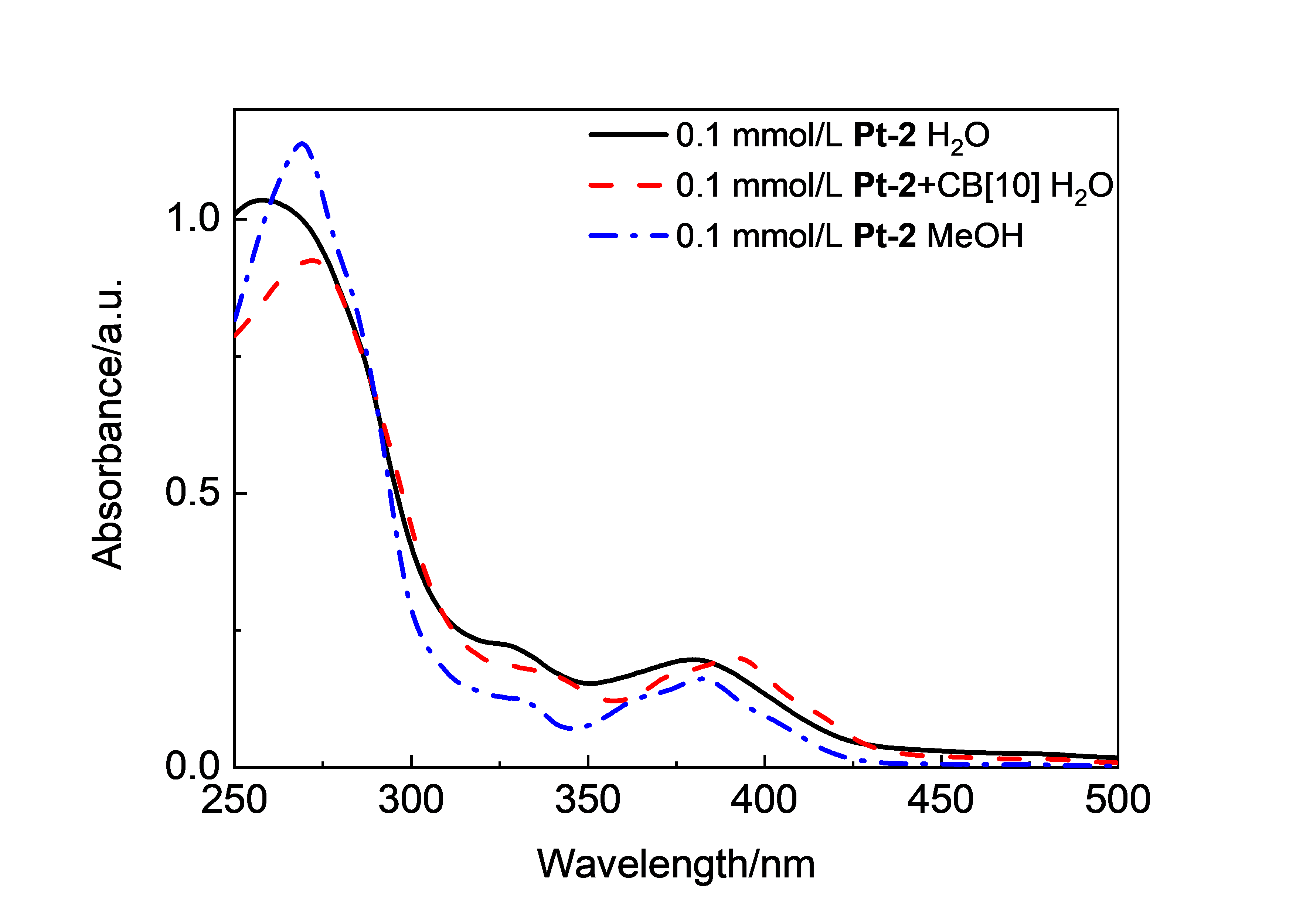
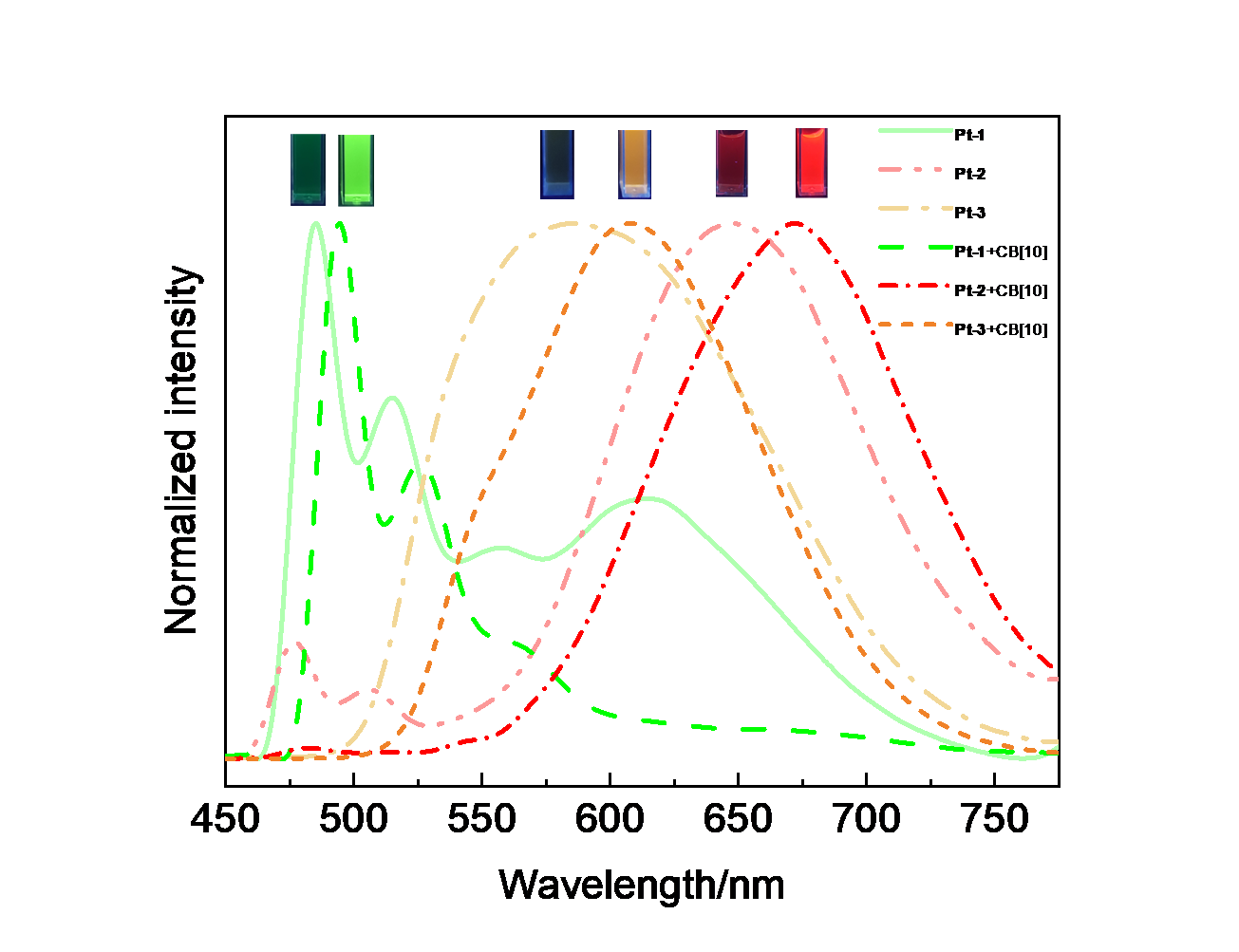
| Complex | λmaxa/nm | τ0/μs | φem/% |
|---|---|---|---|
| Pt-1 | 485 | 13.01 | 2.2 |
| Pt-1+CB[10] | 495 | 20.89 | 16.6 |
| Pt-2 | 648 | 14.64 | 2.7 |
| Pt-2+CB[10] | 667 | 23.74 | 42.0 |
| Pt-3 | 585 | 19.78 | 1.9 |
| Pt-3+CB[10] | 608 | 22.59 | 5.3 |
| Complex | λmaxa/nm | τ0/μs | φem/% |
|---|---|---|---|
| Pt-1 | 485 | 13.01 | 2.2 |
| Pt-1+CB[10] | 495 | 20.89 | 16.6 |
| Pt-2 | 648 | 14.64 | 2.7 |
| Pt-2+CB[10] | 667 | 23.74 | 42.0 |
| Pt-3 | 585 | 19.78 | 1.9 |
| Pt-3+CB[10] | 608 | 22.59 | 5.3 |
| [1] |
(a) Wong, K. M.-C.; Yam, V. W.-W. Acc. Chem. Res. 2011, 44, 424.
doi: 10.1021/ar100130j |
|
(b) Lai, P.-N.; Brysacz, C. H.; Alam, M. K.; Ayoub, N. A.; Gray, T. G.; Bao, J.; Teets, T. S. J. Am. Chem. Soc. 2018, 140, 10198.
doi: 10.1021/jacs.8b04841 |
|
| [2] |
(a) Mauro, M.; Aliprandi, A.; Septiadi, D.; Kehr, N. S.; De Cola, L. Chem. Soc. Rev. 2014, 43, 4144.
doi: 10.1039/C3CS60453E pmid: 25359275 |
|
(b) Chan, A. K.-W.; Ng, M.; Wong, Y.-C.; Chan, M.-Y.; Wong, W.-T.; Yam, V. W.-W. J. Am. Chem. Soc. 2017, 139, 10750.
doi: 10.1021/jacs.7b04952 pmid: 25359275 |
|
|
(c) Lin, J.; Zou, C.; Zhang, X.; Gao, Q.; Suo, S.; Zhuo, Q.; Chang, X.; Xie, M.; Lu, W. Dalton Trans. 2019, 48, 10417.
doi: 10.1039/C9DT02525A pmid: 25359275 |
|
|
(d) Allampally, N. K.; Bredol, M.; Strassert, C. A.; De Cola, L. Chem.-Eur. J. 2014, 20, 16863.
doi: 10.1002/chem.201403772 pmid: 25359275 |
|
| [3] |
Li, K.; Ming Tong, G. S.; Wan, Q.; Cheng, G.; Tong, W.-Y.; Ang, W.-H.; Kwong, W.-L.; Che, C.-M. Chem. Sci. 2016, 7, 1653.
doi: 10.1039/C5SC03766B |
| [4] |
Chow, P.-K.; To, W.-P.; Low, K.-H.; Che, C.-M. Chem.-Asian J. 2014, 9, 534.
doi: 10.1002/asia.201301059 |
| [5] |
Wang, D.; Chen, Y.; Liu, X.; Bian, J.; Yin, X.; Teng, M.; Rong, M.; Wang, Z. Chin. J. Inorg. Chem. 2021, 37, 33. (in Chinese)
|
|
(王登强, 陈宇, 刘小庆, 卞健健, 尹新颖, 滕明瑜, 戎梅竹, 汪正良, 无机化学学报, 2021, 37, 33.)
|
|
| [6] |
Xu, G; Li, J.; Chen, Z. Acta Chim. Sinica 2014, 72, 667. (in Chinese)
doi: 10.6023/A14030217 |
|
(徐广涛, 李佳, 陈忠宁, 化学学报, 2014, 72, 667.)
doi: 10.6023/A14030217 |
|
| [7] |
Zhang, S.; Wang, S.; Zhou, X.; Zhang, H.; Shao, S.; Li, C. Acta Chim. Sinica 2008, 66, 841. (in Chinese)
|
|
(张首才, 王嵩, 周欣, 张红星, 邵琛, 李传碧, 化学学报, 2008, 66, 841.)
|
|
| [8] |
(a) Sun, C.-Y.; To, W.-P.; Hung, F.-F.; Wang, X.-L.; Su, Z.-M.; Che, C.-M. Chem. Sci. 2018, 9, 2357.
doi: 10.1039/C7SC04528J |
|
(b) Li, Z.; Han, Y.; Gao, Z.; Wang, F. ACS Catal. 2017, 7, 4676.
doi: 10.1021/acscatal.7b00709 |
|
| [9] |
(a) Baggaley, E.; Botchway, S. W.; Haycock, J. W.; Morris, H.; Sazanovich, I. V.; Williams, J. A. G.; Weinstein, J. A. Chem. Sci. 2014, 5, 879.
doi: 10.1039/C3SC51875B |
|
(b) Ouyang, C.; Li, Y.; Rees, T. W.; Liao, X.; Jia, J.; Chen, Y.; Zhang, X.; Ji, L.; Chao, H. Angew. Chem., nt. Ed. 2021, 60, 4150.
|
|
|
(c) Law, A. S.-Y.; Lee, L. C.-C.; Lo, K. K.-W.; Yam, V. W.-W. J. Am. Chem. Soc. 2021, 143, 5396.
doi: 10.1021/jacs.0c13327 |
|
| [10] |
(a) Scoditti, S.; Dabbish, E.; Russo, N.; Mazzone, G.; Sicilia, E. Inorg. Chem. 2021, 60, 10350.
doi: 10.1021/acs.inorgchem.1c00822 |
|
(b) Ramu, V.; Gautam, S.; Garai, A.; Kondaiah, P.; Chakravarty, A. R. Inorg. Chem. 2018, 57, 1717.
doi: 10.1021/acs.inorgchem.7b02249 |
|
| [11] |
(a) Summa, G. M.; Scott, B. A. Inorg. Chem. 1980, 19, 1079.
doi: 10.1021/ic50206a064 |
|
(b) Yip, H.-K.; Cheng, L.-K.; Cheung, K.-K.; Che, C.-M. J. Chem. Soc., Dalton Trans. 1993, 2933.
|
|
| [12] |
(a) Yam, V. W.-W.; Tang, R. P.-L.; Wong, K. M.-C.; Cheung, K.-K. Organometallics 2001, 20, 4476.
doi: 10.1021/om010336x |
|
(b) Du, P.; Schneider, J.; Jarosz, P.; Eisenberg, R. J. Am. Chem. Soc. 2006, 128, 7726.
doi: 10.1021/ja0610683 |
|
|
(c) Yang, Z.; Tian, Y.; Li, Z.; Ao, L.; Gao, Z.; Wang, F. Acta Polym. Sin. 2017, 48, 121. (in Chinese)
|
|
|
(杨支帅, 田玉奎, 李子健, 敖雷, 高宗春, 汪峰, 高分子学报, 2017, 48, 121.)
|
|
| [13] |
(a) Yam, V. W.-W.; Wong, K. M.-C.; Zhu, N. J. Am. Chem. Soc. 2002, 124, 6506.
doi: 10.1021/ja025811c |
|
(b) Yam, V. W.-W.; Chan, K. H.-Y.; Wong, K. M.-C.; Zhu, N. Chem.-Eur. J. 2005, 11, 4535.
doi: 10.1002/chem.200500106 |
|
| [14] |
Williams, J. A. G. Chem. Soc. Rev. 2009, 38, 1783.
doi: 10.1039/b804434c pmid: 19587968 |
| [15] |
(a) Hu, S.-J.; Guo, X.-Q.; Zhou, L.-P.; Yan, D.-N.; Cheng, P.-M.; Cai, L.-X.; Li, X.-Z.; Sun, Q.-F. J. Am. Chem. Soc. 2022, 144, 4244.
doi: 10.1021/jacs.2c00760 |
|
(b) Gemen, J.; Ahrens, J.; Shimon, L. J. W.; Klajn, R. J. Am. Chem. Soc. 2020, 142, 17721.
doi: 10.1021/jacs.0c08589 |
|
|
(c) Benson, C. R.; Kacenauskaite, L.; VanDenburgh, K. L.; Zhao, W.; Qiao, B.; Sadhukhan, T.; Pink, M.; Chen, J.; Borgi, S.; Chen, C.-H.; Davis, B. J.; Simon, Y. C.; Raghavachari, K.; Laursen, B. W.; Flood, A. H. Chem 2020, 6, 1978.
doi: 10.1016/j.chempr.2020.06.029 |
|
| [16] |
(a) Liu, S.; Zavalij, P. Y.; Isaacs, L. J. Am. Chem. Soc. 2005, 127, 16798.
doi: 10.1021/ja056287n |
|
(b) Yang, X.; Liu, F.; Zhao, Z.; Liang, F.; Zhang, H.; Liu, S. Chin. Chem. Lett. 2018, 29, 1560.
doi: 10.1016/j.cclet.2018.01.032 |
|
|
(c) Tian, X.; Zuo, M.; Niu, P.; Wang, K.; Hu, X. Chin. J. Org. Chem. 2020, 40, 1823. (in Chinese)
doi: 10.6023/cjoc202003066 |
|
|
(田雪琪, 左旻瓒, 牛蓬勃, 王开亚, 胡晓玉, 有机化学, 2020, 40, 1823.)
doi: 10.6023/cjoc202003066 |
|
| [17] |
(a) Anis-Ul-Haque, K. M.; Woodward, C. E.; Day, A. I.; Wallace, L. Inorg. Chem. 2020, 59, 3942.
doi: 10.1021/acs.inorgchem.9b03603 pmid: 32578987 |
|
(b) Luis, E. T.; Day, A. I.; König, B.; Beves, J. E. Inorg. Chem. 2020, 59, 9135.
doi: 10.1021/acs.inorgchem.0c00986 pmid: 32578987 |
|
|
(c) Zhang, Y.; Liu, M.; Karatchevtseva, I.; Price, J. R.; Tao, Z.; Wei, G. New J. Chem. 2020, 44, 18208.
doi: 10.1039/D0NJ03962D pmid: 32578987 |
|
| [18] |
(a) Kuang, S.; Hu, Z.; Zhang, H.; Zhang, X.; Liang, F.; Zhao, Z.; Liu, S. Chem. Commun. 2018, 54, 2169.
doi: 10.1039/C8CC00593A |
|
(b) Deng, Y.; Yin, H.; Zhao, Z.; Wang, R.; Liu, S. Supramol. Chem. 2018, 30, 706.
doi: 10.1080/10610278.2018.1455977 |
|
| [19] |
Hu, Z.; Sun, D.; Han, X.; Liu, S. Chin. J. Org. Chem. 2020, 40, 1361. (in Chinese)
doi: 10.6023/cjoc201912014 |
|
(胡智雄, 孙冬冬, 韩勰, 刘思敏, 有机化学, 2020, 40, 1361.)
doi: 10.6023/cjoc201912014 |
|
| [20] |
(a) Chen, Y.; Li, K.; Lu, W.; Chui, S. S.-Y.; Ma, C.-W.; Che, C.-M. Angew. Chem., Int. Ed. 2009, 48, 9909.
doi: 10.1002/anie.200905678 pmid: 14686833 |
|
(b) Williams, J. A. G.; Beeby, A.; Davies, E. S.; Weinstein, J. A.; Wilson, C. Inorg. Chem. 2003, 42, 8609.
pmid: 14686833 |
|
|
(c) Cárdenas, D. J.; Echavarren, A. M.; Ramírez de Arellano, M. C. Organometallics 1999, 18, 3337.
doi: 10.1021/om990125g pmid: 14686833 |
|
|
(d) Wang, Z.; Turner, E.; Mahoney, V.; Madakuni, S.; Groy, T.; Li, J. Inorg. Chem. 2010, 49, 11276.
doi: 10.1021/ic100740e pmid: 14686833 |
|
| [21] |
Wan, Q.; Xiao, X.-S.; To, W.-P.; Lu, W.; Chen, Y.; Low, K.-H.; Che, C.-M. Angew. Chem., nt. Ed. 2018, 57, 17189.
|
| [22] |
(a) Chen, Y.; Lu, W.; Che, C.-M. Organometallics 2013, 32, 350.
doi: 10.1021/om300965b |
|
(b) Ai, Y.; Chan, M. H.-Y.; Chan, A. K.-W.; Ng, M.; Li, Y.; Yam, V. W.-W. Proc. Natl. Acad. Sci. U. S. A. 2019, 116, 13856.
doi: 10.1073/pnas.1908034116 |
|
| [23] |
Li, B.; Li, Y.; Chan, M. H.-Y.; Yam, V. W.-W. J. Am. Chem. Soc. 2021, 143, 21676.
doi: 10.1021/jacs.1c10943 |
| [24] |
Xie, M.; Lu, W. Dalton Trans. 2019, 48, 1275.
doi: 10.1039/c8dt03707h pmid: 30608080 |
| [25] |
Zhu, S.; Hu, J.; Zhai, S.; Wang, Y.; Xu, Z.; Liu, R.; Zhu, H. Inorg. Chem. Front. 2020, 7, 4677.
doi: 10.1039/D0QI00735H |
| [26] |
Han, X.; Sun, D.; Tang, S.; Wu, Y.; Wang, L.; Zhang, X.; Liu, S. J. Mater. Chem. C 2021, 9, 17307.
doi: 10.1039/D1TC04433H |
| [27] |
(a) Kozhevnikov, V. N.; Donnio, B.; Bruce, D. W. Angew. Chem., nt. Ed. 2008, 47, 6286.
|
|
(b) Choi, S. J.; Kuwabara, J.; Nishimura, Y.; Arai, T.; Kanbara, T. Chem. Lett. 2011, 41, 65.
doi: 10.1246/cl.2012.65 |
|
|
(c) Abe, T.; Itakura, T.; Ikeda, N.; Shinozaki, K. Dalton Trans. 2009, 711.
|
|
|
(d) Zhang, X.-P.; Mei, J.-F.; Lai, J.-C.; Li, C.-H.; You, X.-Z. J. Mater. Chem. C 2015, 3, 2350.
doi: 10.1039/C4TC02800G |
| [1] | Xuelu Ma, Meng Li, Ming Lei. Trinuclear Transition Metal Complexes in Catalytic Reactions [J]. Acta Chimica Sinica, 2023, 81(1): 84-99. |
| [2] | Qi Meiwei, Liu Yong, Zhou Yongfeng. A Supramolecular Janus Hyperbranched Polymer and Its Electrochemically Responsive Self-Assembly Behavior [J]. Acta Chimica Sinica, 2020, 78(6): 528-533. |
| [3] | Jiao Yang, Zhang Xi. Supramolecular Free Radicals: Fabrication, Modulation and Functions [J]. Acta Chim. Sinica, 2018, 76(9): 659-665. |
| [4] | Cui Bin-Bin, Tang Jian-Hong, Zhong Yu-Wu. Resistive Memory Materials Based on Transition-Metal Complexes [J]. Acta Chim. Sinica, 2016, 74(9): 726-733. |
| [5] | Li Huimei, Wang Jie, Ni Yunzhou, Zhou Yongfeng, Yan Deyue. Synthesis of a Linear-Hyperbranched Supramolecular Polymer and Its Light-Responsive Self-Assembly Behavior [J]. Acta Chim. Sinica, 2016, 74(5): 415-421. |
| [6] | Zhang Yiwe, Ma Xuelu, Zhang Xin, Lei Ming. Theoretical Study on N-N Activation by Thiolate-bridged Dinuclear Dinitrogen Transition-metal Complexes [J]. Acta Chim. Sinica, 2016, 74(4): 340-350. |
| [7] | Li Jing, Zeng Yi, Zhang Xiaohui, Yu Tianjun, Chen Jinping, Li Yi. Stepwise Assembly of CB[7] with Coumarin and Viologen Decorated Dendrimers and Regulation of Fluorescence Properties [J]. Acta Chim. Sinica, 2015, 73(8): 826-834. |
| [8] | Yi Junming, Xiao Xin, Zhang Yunqian, Xue Saifeng, Tao Zhu, Zhang Jianxin. Supramolecular Self-Assembly of Cucurbit[8]uril with 2,2’-(Heptane-1,7-dily) Dibenzimidazolium Chloride [J]. Acta Chimica Sinica, 2014, 72(8): 949-955. |
| [9] | Yue Shiyu, Zhou Yujuan, Yao Yong, Xue Min. Pillar[n]arenes: From Synthesis, Host-Guest Chemistry to Self-Assembly Properties and Applications [J]. Acta Chimica Sinica, 2014, 72(10): 1053-1069. |
| [10] | Kong Rui, Shi Dongjian, Liu Rongjin, Wu Chao, Ni Peihong, Chen Mingqing. Preparation and Properties of Supramolecular Aggregation of Dual-Sensitive Cyclodextrins [J]. Acta Chimica Sinica, 2013, 71(11): 1540-1546. |
| [11] | Wu Xiang, Li Mingli, Gong Liuzhu. Asymmetric Relay Catalysis Reaction Consisting of Metal Complex and Chiral Phosphoric Acids [J]. Acta Chimica Sinica, 2013, 71(08): 1091-1100. |
| [12] | Zhang Xiaojun, Liu Shangzhong, Wu Xuemin, Li Shujing. Supramolecular Hydrogels Obtained by Host-Guest Interactions of Cyclodextrin Dimers with Viologen Polymer [J]. Acta Chimica Sinica, 2012, 70(19): 2066-2072. |
| [13] | Wang Xinke, Sit Met-Met, Sun Jiea, Tang Yong, Xie Zuowei. Synthesis, Structure and Ethylene Polymerization Behavior of Group 4 Metal Complexes Bearing Salicylaldaminato Ligands with Appended Donor Functionality [J]. Acta Chimica Sinica, 2012, 70(18): 1909-1916. |
| [14] | Qiu Yixiang, Wang Shuguang. Theoretical Investigations on Tris(pentamethylcyclopentadienyl) Rare Earth Metal Complexes (C5Me5)3Ln (Ln=Sc, Y, La) [J]. Acta Chimica Sinica, 2012, 70(18): 1930-1938. |
| [15] | Zheng Ke, Lin Lili, Feng Xiaoming. Chiral N,N '-Dioxide-Ni(II) Complex Catalyzed Asymmetric Carbonyl-Ene Reaction of Ethyl Trifluoropyruvate [J]. Acta Chimica Sinica, 2012, 70(17): 1785-1790. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||