Acta Chimica Sinica ›› 2023, Vol. 81 ›› Issue (12): 1716-1723.DOI: 10.6023/A23070349 Previous Articles Next Articles
Article
贾彦荣a, 徐凯a, 赵彦英a, 倪华钢a, 吴滢b, 夏敏a,*( )
)
投稿日期:2023-07-20
发布日期:2023-10-31
基金资助:
Yanrong Jiaa, Kai Xua, Yanying Zhaoa, Huagang Nia, Ying Wub, Min Xiaa( )
)
Received:2023-07-20
Published:2023-10-31
Contact:
*E-mail: Supported by:Share
Yanrong Jia, Kai Xu, Yanying Zhao, Huagang Ni, Ying Wu, Min Xia. Research on Emission Behavior and Mechanofluorochromic Activity of Benzo[d]imidazoles with Region-isomerized Push-Pull Groups[J]. Acta Chimica Sinica, 2023, 81(12): 1716-1723.


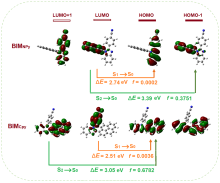

| [1] |
(a) Peng, B.-Y.; Xu, S.-D.; Chi, Z.-G.; Zhang, X.-Q.; Zhang, Y.; Xu, J.-R. Prog. Chem. 2013, 25, 1805 (in Chinese).
|
|
(彭邦银, 许适当, 池振国, 张锡奇, 张艺, 许家瑞, 化学进展, 2013, 25, 1805);
doi: 10.7536/PC130329 |
|
|
(b) Di, B. H.; Chen, Y. L. Chinese Chem. Lett. 2018, 29, 245;
doi: 10.1016/j.cclet.2017.08.043 |
|
|
(c) Yuan, Y,; Yuan, W.; Chen, Y. L. Sci. China Mater. 2016, 59, 507;
doi: 10.1007/s40843-016-5060-7 |
|
|
(d) Yuan, W.; Yuan, Y.; Chen, Y.-L. Acta Polym. Sinica 2016, 11, 1495 (in Chinese).
|
|
|
(袁伟, 袁媛, 陈于蓝, 高分子学报, 2016, 11, 1495);
|
|
|
(e) Yang, J.; Chi, Z.; Zhu, W.; Tang, B. Z.; Li, Z. Sci. China Chem. 2019, 62, 1090;
doi: 10.1007/s11426-019-9512-x |
|
|
(f) Li, Q.; Li, Z. Adv. Sci. 2017, 4, 1600484;
doi: 10.1002/advs.v4.7 |
|
|
(g) Tsuchiya, Y.; Yamaguchi, K.; Miwa, Y.; Kutsumizu, S.; Minoura, M.; Murai, T. Bull. Chem. Soc. Jpn. 2020, 93, 927;
doi: 10.1246/bcsj.20200083 |
|
|
(h) Barman, D.; Gogoi, R.; Narang, K.; Iyer, P. K. Front. Chem. 2020, 8, 483;
doi: 10.3389/fchem.2020.00483 |
|
|
(i) Wang, J.-F.; Li, Z. Acta Chim. Sinica 2021, 79, 575 (in Chinese).
doi: 10.6023/A21010029 |
|
|
(王金凤, 李振, , 化学学报, 2021, 79, 575);
doi: 10.6023/A21010029 |
|
|
(j) Wang, Z.; Liu, L.; Xu, B.; Tian, W. Chem. Res. Chinese Univ. 2021, 37, 100.
doi: 10.1007/s40242-021-0431-0 |
|
| [2] |
(a) Shi, P.; Duan, Y.; Wei, W.; Xu, Z.; Li, Z.; Han, T. J. Mater. Chem. C 2018, 6, 2476;
doi: 10.1039/C7TC05683D |
|
(b) Feng, C.; Wang, K.; Xu, Y.; Liu, L.; Zou, B.; Lu, P. Chem. Commun. 2016, 52, 3836;
doi: 10.1039/C5CC09152G |
|
|
(c) Wang, L.; Wang, K.; Zou, B.; Ye, K.; Zhang, H.; Wang, Y. Adv. Mater. 2015, 27, 2918;
doi: 10.1002/adma.201500589 |
|
|
(d) Xie, W.-Z.; Zheng, H.-C.; Zheng, Y.-S. J. Mater. Chem. C 2017, 5, 10462.
doi: 10.1039/C7TC02525D |
|
| [3] |
(a) Hirata, S.; Watanabe, T. Adv. Mater. 2006, 18, 2725;
doi: 10.1002/adma.v18:20 |
|
(b) Lim, S. J.; An, B. K.; Jung, S. D.; Chung, M. A.; Park, S. Y. Angew. Chem., Int. Ed. 2004, 43, 6346;
doi: 10.1002/anie.v43:46 |
|
|
(c) Olson, C. E.; Previte, M. J. R.; Fourkas, J. T. Nat. Mater. 2002, 1, 225;
doi: 10.1038/nmat766 |
|
|
(d) Irie, M.; Fukaminato, T.; Sasaki, T.; Tamai, N.; Kawai, T. Nature 2002, 420, 759.
doi: 10.1038/420759a |
|
| [4] |
(a) Kishimura, A.; Yamashita, T.; Yamaguchi, K.; Aida, T. Nat. Mater. 2005, 4, 546;
pmid: 15965481 |
|
(b) Zhu, X.; Liu, R.; Li, Y.; Huang, H.; Wang, Q.; Wang, D.; Zhu, X.; Liu, S.; Zhu, H. Chem. Commun. 2014, 50, 12951;
doi: 10.1039/C4CC05913A pmid: 15965481 |
|
|
(c) Qi, Q.; Liu, Y.; Fang, X.; Zhang, Y.; Chen, P.; Wang, Y.; Yang, B.; Xu, B.; Tian, W.; Zhang, S. X. RSC Adv. 2013, 3, 7996;
doi: 10.1039/c3ra40734a pmid: 15965481 |
|
|
(d) Kumar, P.; Dwivedi, J.; Gupta, B. K. J. Mater. Chem. C, 2014, 2, 10468;
doi: 10.1039/C4TC02065K pmid: 15965481 |
|
|
(e) Lu, X.-L.; Xia, M. J. Mater. Chem. C, 2016, 4, 9350.
doi: 10.1039/C6TC02618D pmid: 15965481 |
|
| [5] |
(a) Yuan, W. Z.; Tan, Y.; Gong, Y.; Lu, P.; Lam, J. W. Y.; Shen, X. Y.; Feng, C.; Sung, H. Y.; Lu, Y.; Williams, I. D.; Sun, J. Z.; Zhang, Y.; Tang, B. Z. Adv. Mater. 2013, 25, 2837;
doi: 10.1002/adma.v25.20 |
|
(b) Li, C.; Tang, X.; Zhang, L.; Li, C.; Liu, Z.; Bo, Z.; Dong, Y. Q.; Tian, Y.-H.; Dong, Y.; Tang, B. Z. Adv. Optical Mater. 2015, 3, 1184;
doi: 10.1002/adom.v3.9 |
|
|
(c) Naeem, K. C.; Subhakumari, A.; Varughese, S.; Nair, V. C. J. Mater. Chem. C 2015, 3, 10225;
doi: 10.1039/C5TC02062J |
|
|
(d) Sun, J.; Han, J.; Liu, Y.; Duan, Y.; Han, T.; Yuan, J. J. Mater. Chem. C 2016, 4, 8276;
doi: 10.1039/C6TC03428D |
|
|
(e) Xue, P.; Yang, Z.; Chen, P. J. Mater. Chem. C 2018, 6, 4994.
doi: 10.1039/C8TC01379A |
|
| [6] |
(a) Sagara, Y.; Kato, T. Angew. Chem. Int. Ed. 2011, 50, 9128;
doi: 10.1002/anie.v50.39 |
|
(b) Yoon, S.-J.; Chung, J. W.; Gierschner, J.; Kim, K. S.; Choi, M.-G.; Kim, D.; Park, S. Y. J. Am. Chem. Soc. 2010, 132, 13675;
doi: 10.1021/ja1044665 |
|
|
(c) Sun, H.; Liu, S.; Lin, W.; Zhang, K. Y.; Lv, W.; Huang, W.; Huo, F.; Yang, H.; Jenkins, G.; Zhao, Q.; Huang, W. Nat. Commun. 2014, 5, 3601;
doi: 10.1038/ncomms4601 |
|
|
(d) Zhang, K. Y.; Liu, S.; Zhao, Q.; Huang, W. Coord. Chem. Rev. 2016, 319, 180;
doi: 10.1016/j.ccr.2016.03.016 |
|
|
(e) Chen, X.; Sun, G.; Zhang, T.; Liu, S.; Zhao, Q.; Huang, W. Adv. Mater. 2016, 28, 7137;
doi: 10.1002/adma.v28.33 |
|
|
(f) Zhao, Q.; Xu, W.; Sun, H.; Yang, J.; Zhang, K. Y.; Liu, S.; Ma, Y.; Huang, W. Adv. Opt. Mater. 2016, 4, 1167;
doi: 10.1002/adom.v4.8 |
|
|
(g) Lin, W.; Zhao, Q.; Sun, H.; Zhang, K. Y.; Yang, H.; Yu, Q.; Zhou, X.; Guo, S.; Liu, S.; Huang, W. Adv. Opt. Mater. 2015, 3, 368;
doi: 10.1002/adom.v3.3 |
|
|
(h) Han, J.; Sun, J.; Li, Y.; Duan, Y.; Han, T. J. Mater. Chem. C 2016, 4, 9287.
doi: 10.1039/C6TC03131E |
|
| [7] |
(a) Yang, W.; Yang, Y.; Qiu, Y.; Cao, X.; Huang, Z.; Gong, S.; Yang, C. Mater. Chem. Front. 2020, 4, 2047;
doi: 10.1039/D0QM00247J |
|
(b) Liu, X.-J.; Jiang, H.; Jia, Y.-R.; Xia, M. Dyes & Pigm. 2020, 172, 107845;
|
|
|
(c) Wu, J.; Cheng, Y.; Lan, J.; Wu, D.; Qian, S.; Yan, L.; He, Z.; Li, X.; Wang, K.; Zou, B.; You, J. J. Am. Chem. Soc. 2016, 138, 12803.
doi: 10.1021/jacs.6b03890 |
|
| [8] |
(a) Song, Q.; Wang, Y.; Hu, C.; Zhang, Y.; Sun, J.; Wang, K.; Zhang, C. New J. Chem. 2015, 39, 659;
doi: 10.1039/C4NJ01492H |
|
(b) Ekbote, A.; Mobin, S. M.; Misra, R. J. Mater. Chem. C 2020, 8, 3589;
doi: 10.1039/C9TC05192A |
|
|
(c) Li, W.; Wang, L.; Zhang, J. P. Wang, H. J. Mater. Chem. C 2014, 2, 1887;
doi: 10.1039/c3tc31974a |
|
|
(d) Jiao, Y.; Li, M.; Wang, N.; Lu, T.; Zhou, L.; Huang, Y.; Lu, Z.; Luo, D.; Pu, X. J. Mater. Chem. C 2016, 4, 4269.
doi: 10.1039/C6TC00153J |
|
| [9] |
(a) Liu, X.-J.; Jiang, H.; Jia, Y.-R.; Xia, M. RSC Adv. 2020, 10, 23187;
doi: 10.1039/D0RA02737E |
|
(b) Gao, G.-L.; Jia, Y.-R.; Jiang, H.; Xia, M. Dyes & Pigm. 2021, 186, 109030;
|
|
|
(c) Jia, Y.-R.; Jiang, H.; Gao, G.-L.; Xu, K.; Xia, M. Dyes & Pigm. 2021, 194, 109541;
|
|
|
(d) Liu, X.-J.; Jia, Y.-R.; Jiang, H.; Gao, G.-L.; Xia, M. Acta Chim. Sinica 2019, 77, 1194 (in Chinese).
doi: 10.6023/A19080306 |
|
|
(刘笑静, 贾彦荣, 江豪, 高贯雷, 夏敏, 化学学报, 2019, 77, 1194);
doi: 10.6023/A19080306 |
|
|
(e) Wang, Z.-Y.; Zhao, J.-W.; Li, P.; Feng, T.; Wang, W.-J.; Tao, S.-L.; Tong, Q.-X. New J. Chem. 2018, 42, 8924;
doi: 10.1039/C8NJ01006D |
|
|
(f) Jadhav, T.; Choi, J. M.; Dhokale, B.; Mobin, S. M.; Lee, J. Y.; Misra, R. J. Phys. Chem. C, 2016, 120, 18487;
doi: 10.1021/acs.jpcc.6b06277 |
|
|
(g) Jadhav, T.; Choi, J. M.; Shinde, J.; Lee, J. Y.; Misra, R. J. Mater. Chem. C 2017, 5, 6014;
doi: 10.1039/C7TC00950J |
|
|
(h) Ekbote, A.; Han, S. H.; Jadhav, T.; Mobin, S. M.; Lee, J. Y.; Misra, R. J. Mater. Chem. C 2018, 6, 2077;
doi: 10.1039/C7TC05450E |
|
|
(i) Zhan, Y.; Xu, Y.; Jin, Z.; Ye, W.; Yang, P. Dyes & Pigm. 2017, 140, 452;
|
|
|
(j) Gao, Z.; Wang, K.; Liu, F.; Feng, C.; He, X.; Li, J.; Yang, B.; Zou, B.; Lu, P. Chem. Eur. J. 2017, 23, 773;
doi: 10.1002/chem.v23.4 |
|
|
(k) Nagai, S.; Yamashita, M.; Tachikawa, T.; Ubukata, T.; Asami, M.; Ito, S. J. Mater. Chem. C 2019, 7, 4988;
doi: 10.1039/C9TC00157C |
|
|
(l) Lu, G.; Luo, N.; Hu, F.; Ban, Z.; Zhan, Z.; Huang, G.-S. Adv. Syn. Cat. 2020, 362, 487.
doi: 10.1002/adsc.v362.3 |
|
| [10] |
(a) Zhang, S.; Huang, Y.; Kong, L.; Zhang, X.; Yang, J. Dyes Pigm. 2020, 181, 108574;
doi: 10.1016/j.dyepig.2020.108574 |
|
(b) Liu, X.-J.; Hao, J.; Jia, Y. R.; Xia, M. Dyes Pigm. 2020, 172, 107845;
doi: 10.1016/j.dyepig.2019.107845 |
|
|
(c) Ekbote, A.; Han, S. H.; Jadhav, T.; Mobin, S. M.; Lee, J. Y.; Misra, R. J. Mater. Chem. C 2018, 6, 2077.
doi: 10.1039/C7TC05450E |
|
| [11] |
(a) Sarma, P.; Patir, K.; Sarmah, K. K.; Gogoi, S. K.; Thakuria, R.; Das, P. J. Acta Cryst. 2019, B75, 775;
|
|
(b) Yakir, H. R.; Shimon, L.; Gidron, W. O. Helv. Chim. Acta 2019, 102, e1900027;
doi: 10.1002/hlca.v102.5 |
|
|
(c) Nagura, K.; Saito. S. J. Am. Chem. Soc. 2013, 135, 10322;
doi: 10.1021/ja4055228 |
|
|
(d) Guo, K. P.; Zhang, F.; Guo, S.; Li, K. Chem. Commun. 2017, 53, 1309.
doi: 10.1039/C6CC09186E |
|
| [12] |
(a) Patra, A.; Hebalkar, N.; Sreedhar, B.; Sarkar, M.; Samanta, A.; Radhakrishnan, T. P. Small 2006, 2, 650;
pmid: 23076763 |
|
(b) Chandaluri, C. G.; Radhakrishnan, T. P. Angew. Chem., Int. Ed. 2012, 51, 11849
doi: 10.1002/anie.201205081 pmid: 23076763 |
|
|
(c) Anthony, S. P.; Draper, S. M. J. Phys. Chem. C, 2010, 114, 11708;
doi: 10.1021/jp100594w pmid: 23076763 |
|
|
(d) Molla, M. R.; Gehrig, D.; Roy, L.; Kamm, V.; Paul, A.; Laquai, F.; Ghosh, S. Chem.-Eur. J. 2014, 20, 760;
doi: 10.1002/chem.v20.3 pmid: 23076763 |
|
|
(e) Fernández-Mato, A.; Svnchez-Andújar, M.; Pato-Doldán, B.; Señarís-Rodríguez, M. A.; Platas-Iglesias, C.; Tordera, D.; Bolink, H. J.; Quintela, M.; Peinador, C.; García, M. D. Cryst. Growth Des. 2014, 14, 3849;
doi: 10.1021/cg500379r pmid: 23076763 |
|
|
(f) Fu, H.-Y.; Liu, X.-J.; Xia, M. RSC Adv. 2017, 7, 50720.
doi: 10.1039/C7RA10432D pmid: 23076763 |
| [1] | Jinfeng Wang, Zhen Li. Significant Influence of Molecular Packing in Aggregates on Optoelectronic Properties [J]. Acta Chimica Sinica, 2021, 79(5): 575-587. |
| [2] | Liu Xiaojing, Jia Yanrong, Jiang Hao, Gao Guanlei, Xia Min. Two Polymorphs of Triphenylamine-substituted Benzo[d]imidazole: Mechanoluminescence with Different Colors and Mechanofluorochromism with Emission Shifts in Opposite Direction [J]. Acta Chimica Sinica, 2019, 77(11): 1194-1202. |
| [3] | Mao Wengang, Chen Kang, Ouyang Mi, Sun Jingwei, Zhou Yongbin, Song Qingbao, Zhang Cheng. Synthesis and Characterization of New Cyanostilbene-Based Compound Exhibiting Reversible Mechanochromism [J]. Acta Chimica Sinica, 2013, 71(04): 613-618. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||