Acta Chimica Sinica ›› 2024, Vol. 82 ›› Issue (2): 119-125.DOI: 10.6023/A23110487 Previous Articles Next Articles
Special Issue: 有机氟化学合集
Communication
邓沈娜a,b, 彭常春c, 牛云宏a,b, 许云c, 张云霄a,b, 陈祥a,b, 王红敏c, 刘珊珊a,b, 沈晓a,b,*( )
)
投稿日期:2023-11-02
发布日期:2023-12-20
基金资助:
Shenna Denga,b, Changchun Pengc, Yunhong Niua,b, Yun Xuc, Yunxiao Zhanga,b, Xiang Chena,b, Hongmin Wangc, Shanshan Liua,b, Xiao Shena,b( )
)
Received:2023-11-02
Published:2023-12-20
Contact:
E-mail: Supported by:Share
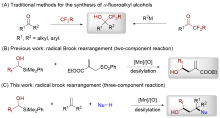
Shenna Deng, Changchun Peng, Yunhong Niu, Yun Xu, Yunxiao Zhang, Xiang Chen, Hongmin Wang, Shanshan Liu, Xiao Shen. Radical Brook Rearrangement Mediated Olefin Difunctionalization Involving α-Fluoroalkyl-α-silyl Methanols[J]. Acta Chimica Sinica, 2024, 82(2): 119-125.
| Entry | Catalyst | Conv./% of 1a | Yield/% of 4a' | Yield/% of 5a' |
|---|---|---|---|---|
| 1 | Mn(OAc)2•4H2O | 100 | 67 | 17 |
| 2 | Mn(acac)2•2H2O | 100 | 48 | 36 |
| 3 | MnCl2•4H2O | 100 | 59 | 16 |
| 4 | MnF2 | 20 | 5 | 0 |
| 5 | MnBr2 | 100 | 13 | 68 |
| 6 | Mn(OAc)2 | 100 | 71 | 19 |
| 7 | Mn(acac)3 | 100 | 53 | 10 |
| 8b | Mn(OAc)2 | 100 | 75 | 18 |
| 9c | Mn(OAc)2 | 100 | 76 (77d) | 18 |
| 10 | Without catalyst | 0 | 0 | 0 |
| Entry | Catalyst | Conv./% of 1a | Yield/% of 4a' | Yield/% of 5a' |
|---|---|---|---|---|
| 1 | Mn(OAc)2•4H2O | 100 | 67 | 17 |
| 2 | Mn(acac)2•2H2O | 100 | 48 | 36 |
| 3 | MnCl2•4H2O | 100 | 59 | 16 |
| 4 | MnF2 | 20 | 5 | 0 |
| 5 | MnBr2 | 100 | 13 | 68 |
| 6 | Mn(OAc)2 | 100 | 71 | 19 |
| 7 | Mn(acac)3 | 100 | 53 | 10 |
| 8b | Mn(OAc)2 | 100 | 75 | 18 |
| 9c | Mn(OAc)2 | 100 | 76 (77d) | 18 |
| 10 | Without catalyst | 0 | 0 | 0 |
| [1] |
(a) Kirsch, P.Modern Fluoroorganic Chemistry: Synthesis, Reactivity, Applications, Wiley-VCH, Weinheim, 2013.
|
|
(b) Wadas, T. J.; Wong, E. H.; Weisman, G. R.; Anderson, C. J. Chem. Rev. 2010, 110, 2858.
doi: 10.1021/cr900325h |
|
|
(c) Shen, J.; Xu, J.; He, L.; Liang, C.; Li, W. Chin. Chem. Lett. 2022, 33, 1227.
doi: 10.1016/j.cclet.2021.09.005 |
|
| [2] |
(a) Purser, S.; Moore, P. R.; Swallow, S.; Gouverneur, V. Chem. Soc. Rev. 2008, 37, 320.
doi: 10.1039/B610213C pmid: 37695320 |
|
(b) Britton, R.; Gouverneur, V.; Lin, J.-H.; Meanwell, M.; Ni, C.; Pupo, G.; Xiao, J.-C.; Hu, J. Nat. Rev. Methods Primers 2021, 1, 47.
doi: 10.1038/s43586-021-00042-1 pmid: 37695320 |
|
|
(c) Wu, W.; Wang, J.; Wang, Y.; Huang, Y.; Tan, Y.; Weng, Z. Angew. Chem., Int. Ed. 2017, 56, 10476.
doi: 10.1002/anie.v56.35 pmid: 37695320 |
|
|
(d) Yang, J.; Zhu, S.; Wang, F.; Qing, F. L.; Chu, L. Angew. Chem., Int. Ed. 2021, 60, 4300.
doi: 10.1002/anie.v60.8 pmid: 37695320 |
|
|
(e) Zhang, L.; Yan, J.; Ahmadli, D.; Wang, Z.; Ritter, T. J. Am. Chem. Soc. 2023, 145, 20182.
doi: 10.1021/jacs.3c07119 pmid: 37695320 |
|
|
(f) Zhang, M.; Lin, J. H.; Xiao, J. C. Angew. Chem., Int. Ed. 2019, 58, 6079.
doi: 10.1002/anie.v58.18 pmid: 37695320 |
|
|
(g) Zhao, H.; Leng, X. B.; Zhang, W.; Shen, Q. Angew. Chem., Int. Ed. 2022, 61, e202210151.
doi: 10.1002/anie.v61.42 pmid: 37695320 |
|
|
(h) Zhao, S.; Guo, Y.; Su, Z.; Cao, W.; Wu, C.; Chen, Q.-Y. Org. Lett. 2020, 22, 8634.
doi: 10.1021/acs.orglett.0c03238 pmid: 37695320 |
|
|
(i) Li, S.; Lu, J. X.; Liu, J.; Jiang, L. Q.; Yi, W. B. Acta Chim. Sinica 2023, 81, doi: 10.6023/A23080386. (in Chinese)
pmid: 37695320 |
|
|
(李珊, 路俊欣, 刘杰, 蒋绿齐, 易文斌, 化学学报, 2023, 81, doi: 10.6023/A23080386)
pmid: 37695320 |
|
|
(j) Hu, J.; Zeng, Y. Synthesis 2016, 48, 2137.
doi: 10.1055/s-00000084 pmid: 37695320 |
|
| [3] |
(a) Yamazaki, T.; Haga, J.; Kitazume, T. Bioorg. Med. Chem. Lett. 1991, 1, 271.
doi: 10.1016/S0960-894X(01)81041-6 |
|
(b) Gauthier, J. Y.; Li, C. S.; Mellon, C. US 20060111440, 2006.
|
|
|
(c) Wouters, J.; Moureau, F.; Evrard, G.; Koenig, J.-J.; Jegham, S.; George, P.; Durant, F. Biorg. Med. Chem. 1999, 7, 1683.
doi: 10.1016/S0968-0896(99)00102-9 |
|
|
(d) Biadatti, T.; Thoreau, E.; Voegel, J.; Jomard, A. WO 2004020379, 2004.
|
|
| [4] |
(a) Du, G.-F.; Wang, Y.; Gu, C.-Z.; Dai, B.; He, L. RSC Adv. 2015, 5, 35421.
doi: 10.1039/C5RA04472C |
|
(b) Linclau, B.; Wang, Z.; Compain, G.; Paumelle, V.; Fontenelle, C. Q.; Wells, N.; Weymouth‐Wilson, A. Angew. Chem., Int. Ed. 2016, 55, 674.
doi: 10.1002/anie.v55.2 |
|
|
(c) Ni, C.; Hu, J. Tetrahedron Lett. 2005, 46, 8273.
doi: 10.1016/j.tetlet.2005.09.162 |
|
|
(d) Song, J. J.; Tan, Z.; Reeves, J. T.; Gallou, F.; Yee, N. K.; Senanayake, C. H. Org. Lett. 2005, 7, 2193.
doi: 10.1021/ol050568i |
|
| [5] |
Chen, X.; Gong, X.; Li, Z.; Zhou, G.; Zhu, Z.; Zhang, W.; Liu, S.; Shen, X. Nat. Commun. 2020, 11, 2756.
doi: 10.1038/s41467-020-16380-9 |
| [6] |
Li, Z.; Chen, X.; Peng, C.; Xu, Y.; Wang, H.; Liu, S.; Shen, X. ChemCatChem. 2023, e202301075, doi: 10.1002/cctc.202301075.
|
| [7] |
(a) Jiang, X.; Zhang, M.-M.; Xiong, W.; Lu, L.-Q.; Xiao, W.-J. Angew. Chem., Int. Ed. 2019, 58, 2402.
doi: 10.1002/anie.v58.8 |
|
(b) Properzi, R.; Kaib, P. S. J.; Leutzsch, M.; Pupo, G.; Mitra, R.; De, C. K.; Song, L.; Schreiner, P. R.; List, B. Nat. Chem. 2020, 12, 1174.
doi: 10.1038/s41557-020-00558-1 |
|
|
(c) Tang, S.; Liu, K.; Liu, C.; Lei, A. Chem. Soc. Rev. 2015, 44, 1070.
doi: 10.1039/C4CS00347K |
|
|
(d) Zhu, N.; Zhao, J.; Bao, H. Chem. Sci. 2017, 8, 2081.
doi: 10.1039/C6SC04274K |
|
|
(e) Liwosz, T. W.; Chemler, S. R. Org. Lett. 2013, 15, 3034.
doi: 10.1021/ol401220b |
|
|
(f) Zhang, Z.; Gong, L.; Zhou, X.-Y.; Yan, S.-S.; Li, J.; Yu, D.-G. Acta Chim. Sinica 2019, 77, 783. (in Chinese)
doi: 10.6023/A19060208 |
|
|
(张振, 龚莉, 周晓渝, 颜思顺, 李静, 余达刚, 化学学报, 2019, 77, 783.)
doi: 10.6023/A19060208 |
|
|
(g) Zheng, L.; Wang, Y.; Cai, L.; Guo, W. Chin. J. Org. Chem. 2022, 42, 4078. (in Chinese)
doi: 10.6023/cjoc202208026 |
|
|
(郑绿茵, 王逸涵, 蔡刘欢, 郭维, 有机化学, 2022, 42, 4078.)
doi: 10.6023/cjoc202208026 |
|
| [8] |
Chen, X.; Zhu, Z.; Liu, S.; Chen, Y.-H.; Shen, X. Chin. Chem. Lett. 2022, 33, 2391.
doi: 10.1016/j.cclet.2021.10.083 |
| [9] |
Smith, J. R.; Collins, B. S. L.; Hesse, M. J.; Graham, M. A.; Myers, E. L.; Aggarwal, V. K. J. Am. Chem. Soc. 2017, 139, 9148.
doi: 10.1021/jacs.7b05149 |
| [10] |
(a) Feng, T.; Liu, C.; Wu, Z.; Wu, X.; Zhu, C. Chem. Sci. 2022, 13, 2669.
doi: 10.1039/D2SC00015F |
|
(b) Wang, D.; Ren, R.; Zhu, C. J. Org. Chem. 2016, 81, 8043.
doi: 10.1021/acs.joc.6b01433 |
|
|
(c) Zhang, Y.-H.; Zhang, W.-W.; Zhang, Z.-Y.; Zhao, K.; Loh, T.-P. Org. Lett. 2019, 21, 5101.
doi: 10.1021/acs.orglett.9b01703 |
|
|
(d) Wang, D.; He, Y.; Dai, H.; Huang, C.; Yuan, X. A.; Xie, J. Chin. J. Chem. 2020, 38, 1497.
doi: 10.1002/cjoc.v38.12 |
|
| [11] |
(a) Chuang, T.-H.; Fang, J.-M.; Jiaang, W.-T.; Tsai, Y.-M. J. Org. Chem. 1996, 61, 1794.
doi: 10.1021/jo951980m pmid: 10789438 |
|
(b) Dalton, J. C.; Bourque, R. A. J. Am. Chem. Soc. 1981, 103, 699.
doi: 10.1021/ja00393a049 pmid: 10789438 |
|
|
(c) Deng, Y.; Liu, Q.; Smith, A. B., III. J. Am. Chem. Soc. 2017, 139, 9487.
doi: 10.1021/jacs.7b05165 pmid: 10789438 |
|
|
(d) He, X.; Zhao, Y.; Zhang, Z.; Shen, X. Org. Lett. 2022, 24, 1991.
doi: 10.1021/acs.orglett.2c00428 pmid: 10789438 |
|
|
(e) Li, Z.; Zhang, Y.; Zhang, Y.; He, X.; Shen, X. Angew. Chem., Int. Ed. 2023, 62, e202303218.
doi: 10.1002/anie.v62.20 pmid: 10789438 |
|
|
(f) Paredes, M. D.; Alonso, R. J. Org. Chem. 2000, 65, 2292.
pmid: 10789438 |
|
|
(g) Yang, Z.; Niu, Y.; He, X.; Chen, S.; Liu, S.; Li, Z.; Chen, X.; Zhang, Y.; Lan, Y.; Shen, X. Nat. Commun. 2021, 12, 2131.
doi: 10.1038/s41467-021-22382-y pmid: 10789438 |
|
|
(h) Zhang, Y.; Zhang, Y.; Guo, Y.; Liu, S.; Shen, X. Chem. Catal. 2022, 2, 1380.
pmid: 10789438 |
|
|
(i) Zhang, Y.; Zhang, Y.; Shen, X. Chem. Catal. 2021, 1, 423.
pmid: 10789438 |
|
|
(j) Zhu, Z.; Chen, X.; Liu, S.; Zhang, J.; Shen, X. Eur. J. Org. Chem. 2021, 2021, 4927.
doi: 10.1002/ejoc.v2021.35 pmid: 10789438 |
|
|
(k) Zhang, Y.; Zhang, Y.; Ye, C.; Qi, X.; Wu, L.-Z.; Shen, X. Nat. Commun. 2022, 13, 6111.
doi: 10.1038/s41467-022-33730-x pmid: 10789438 |
|
|
(l) Lin, Q.; Zheng, S.; Chen, L.; Wu, J.; Li, J.; Liu, P.; Dong, S.; Liu, X.; Peng, Q.; Feng, X. Angew. Chem., nt. Ed. 2022, 61, e202203650;
pmid: 10789438 |
|
|
(m) Lin, Q.; Wang, S.; Weng, R.; Cao, W.; Feng, X. Org. Lett. 2023, 25, 6262.
doi: 10.1021/acs.orglett.3c02042 pmid: 10789438 |
|
| [12] |
(a) Wiles, R. J.; Molander, G. A. Isr. J. Chem. 2020, 60, 281.
doi: 10.1002/ijch.v60.3-4 |
|
(b) Yamashita, K.; Fujiwara, Y.; Hamashima, Y. J. Org. Chem. 2023, 88, 1865.
doi: 10.1021/acs.joc.2c02575 |
|
|
(c) Johnson, L. K.; Barnes, G. L.; Fernandez, S. A.; Vanderwal, C. D. Angew. Chem., Int. Ed. 2023, 62, e202303228.
doi: 10.1002/anie.v62.21 |
|
| [13] |
(a) Nakagawa, M.; Matsuki, Y.; Nagao, K.; Ohmiya, H. J. Am. Chem. Soc. 2022, 144, 7953.
doi: 10.1021/jacs.2c00527 |
|
(b) Zhang, Y.-Q.; Jiang, Y.-Q.; Wang, Y.-H.; Qi, C.; Ling, Y.; Zhang, Y.; Liu, G.-Q. J. Org. Chem. 2023, 88, 7431.
doi: 10.1021/acs.joc.3c00788 |
|
|
(c) Rao, W.-H.; Li, Q.; Li, Y.-G.; Jiang, L.-L.; Yue, M.-X.; Zou, G.-D.; Cao, X. Eur. J. Org. Chem. 2023, 26, e202300191.
doi: 10.1002/ejoc.v26.18 |
| [1] | Fan Xuefeng, Zhao Huijun, Zhu Chen. Recent Advances in the Synthesis of Distal Fluorinated Ketones [J]. Acta Chim. Sinica, 2015, 73(10): 979-983. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||