-
-
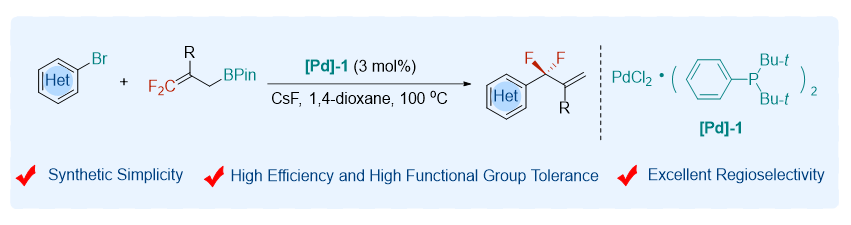
 About the Cover:Zhang, Xingang et al. on page 105-109: An efficient method that can efficiently access medicinally interesting gem-difluoroallylated heteroarenes through palladium-catalyzed cross-coupling of heteroaryl bromides with gem-difluoroallylborons has been developed.
About the Cover:Zhang, Xingang et al. on page 105-109: An efficient method that can efficiently access medicinally interesting gem-difluoroallylated heteroarenes through palladium-catalyzed cross-coupling of heteroaryl bromides with gem-difluoroallylborons has been developed. -
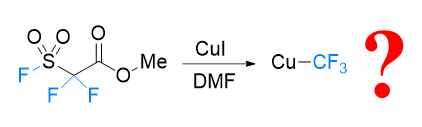
 About the Cover:Xue, Xiao-Song et al. on page 132-137: FSO2CF2CO2Me, known as Chen's reagent, is perhaps the first used trifluoromethylation reagent originate from China in 1989. Based on the experimental results, its detailed mechanism governing the generation of copper(I) trifluoromethyl from Chen's reagent in the presence of a CuI catalyst is revealed by density functional theory (DFT) calculations.
About the Cover:Xue, Xiao-Song et al. on page 132-137: FSO2CF2CO2Me, known as Chen's reagent, is perhaps the first used trifluoromethylation reagent originate from China in 1989. Based on the experimental results, its detailed mechanism governing the generation of copper(I) trifluoromethyl from Chen's reagent in the presence of a CuI catalyst is revealed by density functional theory (DFT) calculations. -
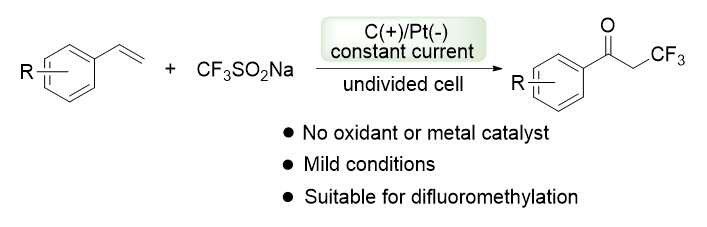
 About the Cover:Yi, Wenbin et al. on page 110-114: Herein we describe an electrochemical synthesis of α-trifluoromethylated ketones from alkenes based on sodium trifluoromethanesulfinate. Sodium trifluoromethanesulfinate generates trifluoromethyl radicals through anodic oxidation to attack the carbon-carbon double bonds of alkenes, and then oxidized in air atmosphere to obtain the target compounds. In addition, this reaction can be applied to the synthesis of α-difluoromethylated ketones when using sodium difluoromethanesulfinate instead of sodium trifluoromethanesulfinate.
About the Cover:Yi, Wenbin et al. on page 110-114: Herein we describe an electrochemical synthesis of α-trifluoromethylated ketones from alkenes based on sodium trifluoromethanesulfinate. Sodium trifluoromethanesulfinate generates trifluoromethyl radicals through anodic oxidation to attack the carbon-carbon double bonds of alkenes, and then oxidized in air atmosphere to obtain the target compounds. In addition, this reaction can be applied to the synthesis of α-difluoromethylated ketones when using sodium difluoromethanesulfinate instead of sodium trifluoromethanesulfinate. -
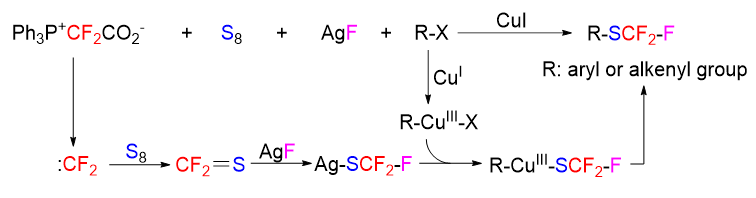
 About the Cover:Shu, Chao et al. on page 171-189: Studies on sultines and their transformations have garnered increasing interest over the past 130 years, leading to a systematic summary of sultines based on synthesis methods, physical properties, and applications. This summary includes a review of synthetic tactics showcasing product diversity, selectivity, and applicability, as well as possible mechanistic explanations. Furthermore, the chemical transformations of sultines, such as photolysis, thermolysis, and various other reactions, alongside their utility in synthesis, medicine, and materials are discussed, offering a future perspective for researchers in the field.
About the Cover:Shu, Chao et al. on page 171-189: Studies on sultines and their transformations have garnered increasing interest over the past 130 years, leading to a systematic summary of sultines based on synthesis methods, physical properties, and applications. This summary includes a review of synthetic tactics showcasing product diversity, selectivity, and applicability, as well as possible mechanistic explanations. Furthermore, the chemical transformations of sultines, such as photolysis, thermolysis, and various other reactions, alongside their utility in synthesis, medicine, and materials are discussed, offering a future perspective for researchers in the field.
-
-
Current Issue
Editorial
Communication
Article
Account
Review