Acta Chimica Sinica ›› 2025, Vol. 83 ›› Issue (1): 45-51.DOI: 10.6023/A24110333 Previous Articles Next Articles
Original article
王跃a,b,†, 邹莹b,†, 张元b, 郑舒婕b, 王恒宇b, 刘天赋b,*( ), 李仁富b,*(
), 李仁富b,*( )
)
投稿日期:2024-11-01
发布日期:2024-12-17
基金资助:
Yue Wanga,b,†, Ying Zoub,†, Yuan Zhangb, Shujie Zhengb, Hengyu Wangb, Tianfu Liub( ), Renfu Lib(
), Renfu Lib( )
)
Received:2024-11-01
Published:2024-12-17
Contact:
*E-mail: About author:Supported by:Share
Yue Wang, Ying Zou, Yuan Zhang, Shujie Zheng, Hengyu Wang, Tianfu Liu, Renfu Li. Zirconium-Based Metal-Organic Frameworks for Oxygen Sensing[J]. Acta Chimica Sinica, 2025, 83(1): 45-51.


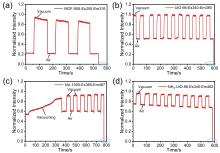



| [1] |
Kitagawa, Y.; Nakai, T.; Hosoya, S.; Shoji, S.; Hasegawa, Y. ChemPlusChem 2023, 88, e202300149.
|
| [2] |
Ellis, J. E.; Sorescu, D. C.; Burkert, S. C.; White, D. L.; Star, A. ACS Appl. Mater. 2013, 9, 27142.
|
| [3] |
Zhao, H. M.; Wang, Q. Q.; Wang, S. M.; Yin, J. Y.; Wang, H. B.; Shao, W. H.; Yao, Z. X.; Yao, J. T.; Zang, L. X. Inorg. Chem. Front. 2023, 10, 5161.
|
| [4] |
Salaris, N.; Chen, W. Q.; Haigh, P.; Caciolli, L.; Giobbe, G. G.; De Coppi, P.; Papakonstantinou, I.; Tiwari, M. K. Biosens. Bioelectron. 2024, 255, 116198.
|
| [5] |
Duong, H. D.; Sohn, O. J.; Rhee, J. I. Biosensors-Basel 2020, 10, 86.
|
| [6] |
Liang, Y. N.; Wu, Z. X.; Wei, Y. N.; Ding, Q. L.; Zilberman, M.; Tao, K.; Xie, X.; Wu, J. Nano-Micro Lett. 2022, 14, 52.
|
| [7] |
Ji, H. Y.; Liu, Y.; Chen, G. L.; Kong, L.; Yuan, Z. Y.; Meng, F. L. Sensor Actuat. B-Chem. 2024, 414, 315884.
|
| [8] |
Biring, S.; Sadhu, A. S.; Deb, M. Sensors 2019, 19, 5124.
|
| [9] |
Ren, T.; Jin, X.; Deng, S. H.; Guo, K.; Gao, Y. H.; Shi, X.; Xu, L.; Bai, X.; Shang, Y. B.; Jin, P. K.; Wang, X. C. J. Clean. Prod. 2024, 434, 140332.
|
| [10] |
Willett, M. Sensors 2014, 14, 6084.
doi: 10.3390/s140406084 pmid: 24681673 |
| [11] |
Papkovsky, D. B.; Dmitriev, R. I. Chem. Soc. Rev. 2013, 42, 8700.
doi: 10.1039/c3cs60131e pmid: 23775387 |
| [12] |
Mirzaei, A.; Kim, J. Y.; Kim, H. W.; Kim, S. S. Acc. Chem. Res. 2024, 57, 2395.
|
| [13] |
Dou, Z. S.; Yu, J. C.; Cui, Y. J.; Yang, Y.; Wang, Z. Y.; Yang, D. R.; Qian, G. D. J. Am. Chem. Soc. 2014, 136, 5527.
|
| [14] |
Xu, R. Y.; Wang, Y. F.; Duan, X. P.; Lu, K. D.; Micheroni, D.; Hu, A. G.; Lin, W. B. J. Am. Chem. Soc. 2016, 138, 2158.
|
| [15] |
Zhu, C. Y.; Wang, Z.; Mo, J. T.; Fan, Y. N.; Pan, M. J. Mater. Chem. C 2020, 8, 9916.
|
| [16] |
Zhao, D.; Cui, Y. J.; Yang, Y.; Qian, G. D. CrystEngComm 2016, 18, 3746.
|
| [17] |
Zhang, Y. M.; Yuan, S.; Day, G.; Wang, X.; Yang, X. Y.; Zhou, H. C. Coord. Chem. Rev. 2018, 354, 28.
|
| [18] |
Zhang, Y. F.; Zhang, Z. H.; Fang, H.; Guo, X. A.; Ma, Y. N.; Zhang, Y. Z.; Xue, D. X. Inorg. Chem. 2023, 62, 20513.
|
| [19] |
Chen, Z. J.; Feng, L.; Liu, L. M.; Bhatt, P. M.; Adil, K.; Emwas, A. H.; Assen, A. H.; Belmabkhout, Y.; Han, Y.; Eddaoudi, M. Langmuir 2018, 34, 14546.
|
| [20] |
Dai, Q. Q.; Yang, Z. F.; Li, J.; Cao, Y.; Tang, H. B.; Wei, X. C. Renew. Energy 2021, 163, 1588.
|
| [21] |
Wang, N.; Ma, L.; Li, Z. X.; Zhou, C. Y.; Su, X. G. Inorg. Chem. Front. 2024, 11, 3820.
|
| [22] |
Huang, N.; Yuan, S.; Drake, H.; Yang, X. Y.; Pang, J. D.; Qin, J. S.; Li, J. L.; Zhang, Y. M.; Wang, Q.; Jiang, D. L.; Zhou, H. C. J. Am. Chem. Soc. 2017, 139, 18590.
doi: 10.1021/jacs.7b09553 pmid: 29172485 |
| [23] |
Zhu, Y. T.; Zhu, L. W.; Song, W. M.; Deng, C. H. Inorg. Chem. Commun. 2023, 154, 110917.
|
| [24] |
Guo, T. T.; Wei, Q. H.; Gao, H. Y.; Chen, J.; Wang, C. A.; Liu, Z. Y.; Li, J.; Wang, G. Mater. Today Chem. 2023, 34, 101693.
|
| [25] |
Sonal, S.; Mishra, B. K. Chem. Eng. J. 2021, 424, 130509.
|
| [26] |
Jia, C.; He, T.; Wang, G. M. Coord. Chem. Rev. 2023, 476, 214930.
|
| [27] |
Tan, J. X.; Chen, Z. Y.; Chen, C. H.; Hsieh, M. F.; Lin, A. Y. C.; Chen, S. S.; Wu, K. C. W. J. Hazard. Mater. 2023, 451, 131113.
|
| [28] |
Li, Z. P.; Geng, J. X.; Liu, Y. Y.; Sun, Z. C.; Wang, Y.; Wang, A. J. Chem. J. Chin. Univ. 2023, 44, 20230059 (in Chinese).
|
|
( 李子鹏, 耿佳鑫, 刘颖雅, 孙志超, 王耀, 王安杰, 高等学校化学学报, 2023, 44, 20230059.)
|
|
| [29] |
Zhang, J.; Xia, T.; Zhao, D.; Cui, Y.; Yang, Y.; Qian, G. Sensor Actuat. B-Chem. 2018, 260, 63.
|
| [30] |
Xia, T. F.; Jiang, L. C.; Zhang, J.; Wan, Y. T.; Yang, Y.; Gan, J. L.; Cui, Y. J.; Yang, Z. M.; Qian, G. D. Microporous Mesoporous Mater. 2020, 305, 110396.
|
| [31] |
Lemon, C. M. Pure Appl. Chem. 2018, 90, 1359.
|
| [32] |
Furukawa, H.; Gándara, F.; Zhang, Y. B.; Jiang, J. C.; Queen, W. L.; Hudson, M. R.; Yaghi, O. M. J. Am. Chem. Soc. 2014, 136, 4369.
|
| [33] |
Mondloch, J. E.; Bury, W.; Fairen-Jimenez, D.; Kwon, S.; DeMarco, E. J.; Weston, M. H.; Sarjeant, A. A.; Nguyen, S. T.; Stair, P. C.; Snurr, R. Q.; Farha, O. K.; Hupp, J. T. J. Am. Chem. Soc. 2013, 135, 10294.
doi: 10.1021/ja4050828 pmid: 23829224 |
| [34] |
Han, Y. T.; Liu, M.; Li, K. Y.; Zuo, Y.; Zhang, G. L.; Zhang, Z. C.; Guo, X. W. Chin. J. Appl. Chem. 2016, 33, 368 (in Chinese).
|
|
( 韩易潼, 刘民, 李克艳, 左轶, 张国亮, 张宗超, 郭新闻, 应用化学, 2016, 33, 368.)
|
|
| [35] |
Duan, Z. G.; Pei, Y.; Zheng, S. S.; Wang, Z. Y.; Wang, Y. G.; Wang, J. J.; Hu, Y.; Lv, C. X.; Zhong, W. Chin. J. Inorg. Chem. 2024, 40, 496 (in Chinese).
|
|
( 段章圭, 裴毅, 郑珊珊, 王召阳, 王勇光, 王骏杰, 胡杨, 吕春欣, 钟伟, 无机化学学报, 2024, 40, 496.)
|
| [1] | Hangqing Lin, Ruoru Ma, Yilan Jiang, Murong Xu, Yangpeng Lin, Kezhao Du. Research Progress of Materials Used for Elemental Halogen Capture [J]. Acta Chimica Sinica, 2024, 82(1): 62-74. |
| [2] | Jianchuan Liu, Cuiyan Li, Yaozu Liu, Yujie Wang, Qianrong Fang. Highly-Stable Two-Dimensional Bicarbazole-based sp2-Carbon-conjugated Covalent Organic Framework for Efficient Electrocatalytic Oxygen Reduction★ [J]. Acta Chimica Sinica, 2023, 81(8): 884-890. |
| [3] | Mengxi Zhang, Xiao Feng. Fabrication Strategies of Conjugated Microporous Polymer Membranes for Molecular Separation [J]. Acta Chimica Sinica, 2022, 80(2): 168-179. |
| [4] | Huang Gang, Chen Yuzhen, Jiang Hailong. Metal-Organic Frameworks for Catalysis [J]. Acta Chimica Sinica, 2016, 74(2): 113-129. |
| [5] | Wu Jinxiong, Liu Zhongfan, Peng Hailin. Fluorescence Quenching Effect of Rhodanmine 6G on Two-Dimensional Bi2Se3 Crystals [J]. Acta Chim. Sinica, 2015, 73(9): 944-948. |
| [6] | Sun Lei, Deng Weiqiao. Progress and Prospect of Theoretical Simulation of Microporous Materials [J]. Acta Chim. Sinica, 2015, 73(6): 579-586. |
| [7] | Li Xiping, Liu Sijia, Wu Zhan, Jiang Jianhui. A Novel Approach to Detect 2,4,6-trinitrotoluene/2,4,6-trinitrophenol Based on Fluorescence Quenching via Charge Transfer of Silicon Quantum Dots [J]. Acta Chimica Sinica, 2014, 72(5): 563-568. |
| [8] | Xiong Jingfang, Xiao Pei, Wu Qiang, Wang Xizhang, Hu Zheng. Synthesis and Gas-Sensing Properties of ZnO Porous Microflowers [J]. Acta Chimica Sinica, 2014, 72(4): 433-439. |
| [9] | Sui Dong, Huang Yi, Huang Lu, Zhang Yi, Chen Yongsheng. Investigation of Gas Storage Properties of Graphene Material Prepared by Microwave-assisted Reduction of Graphene Oxide [J]. Acta Chimica Sinica, 2014, 72(3): 382-387. |
| [10] | Cheng Fangyi, Chen Jun. Nanoporous Catalysts for Rechargeable Li-air Batteries [J]. Acta Chimica Sinica, 2013, 71(04): 473-477. |
| [11] | Shao Yue, Ma Yong. Amino Group Surface-functionalized Ordered Mesoporous Materials:One-pot Synthesis, Heavy-metal Ion and CO2 Adsorption [J]. Acta Chimica Sinica, 2012, 70(18): 1957-1962. |
| [12] | Luo Yi, Chen Tianfeng, Huang Xiaochun, Wang Yi, Wong Yumshing, Zheng Wenjie. Synthesis, Anticancer Activity of a Novel Selenadiazole Derivative and Its Interaction with Bovine Serum Albumin [J]. Acta Chimica Sinica, 2012, 70(11): 1295-1303. |
| [13] | Gan Xiaojuan, Liu Shaopu, Liu Zhongfang, Wang Yaqiong, Cui Zhiping, Hu Xiaoli. Fluorescence Quenching Method for the Determination of Analgin and Metabolin with Some Aromatic Amino Acids as Probes [J]. Acta Chimica Sinica, 2012, 70(01): 58-64. |
| [14] | LIU Lu-Sha, FAN Jun, HU Chun-Mei, SUN Yang, HU Xiao-Yun, ZHAO Ying-Yong, WEI Song, LIANG Xu-Hua. Studies on the Interaction between TubeimosideII and Human Serum Albumin by Spectroscopic Methods [J]. Acta Chimica Sinica, 2011, 69(21): 2589-2596. |
| [15] | WEI Hong, WANG Yun-Yun, SONG Er-Qun. New Method for Detection of Norfloxacin Nicotinate Based on the CdTe Quantum Dots [J]. Acta Chimica Sinica, 2011, 69(17): 2039-2046. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||