Acta Chimica Sinica ›› 2025, Vol. 83 ›› Issue (8): 878-886.DOI: 10.6023/A25040134 Previous Articles Next Articles
Article
尚建宇a,b, 王超超a,b, 高欣冉a,b, 章寅a,b,*( ), 沙菁㛃a,b,*(
), 沙菁㛃a,b,*( )
)
投稿日期:2025-04-27
发布日期:2025-06-17
通讯作者:
章寅, 沙菁㛃
基金资助:
Jianyu Shanga,b, Chaochao Wanga,b, Xinran Gaoa,b, Yin Zhanga,b,*( ), Jingjie Shaa,b,*(
), Jingjie Shaa,b,*( )
)
Received:2025-04-27
Published:2025-06-17
Contact:
Yin Zhang, Jingjie Sha
Supported by:Share
Jianyu Shang, Chaochao Wang, Xinran Gao, Yin Zhang, Jingjie Sha. Conformational Conversion Strategy-Driven Solid-State Nanopore Enables Concentration-Sensitive Quantification of Biomarkers[J]. Acta Chimica Sinica, 2025, 83(8): 878-886.
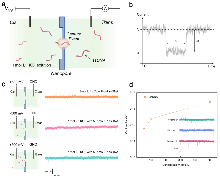









| [1] |
doi: 10.1016/j.cell.2009.01.002 pmid: 19167326 |
| [2] |
|
| [3] |
doi: 10.1038/nrc1840 pmid: 16557279 |
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
doi: 10.1038/labinvest.2010.194 pmid: 21116241 |
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
doi: 10.1038/cr.2008.282 pmid: 18766170 |
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
|
(马超凡, 徐伟, 刘巍, 徐昌晖, 沙菁㛃, 化学学报, 2023, 81, 857).
|
|
| [24] |
|
| [25] |
doi: 10.1038/s41588-022-01024-z pmid: 35379992 |
| [26] |
|
| [27] |
doi: 10.1016/j.ejpb.2023.08.019 pmid: 37666365 |
| [28] |
|
| [29] |
doi: 10.1038/nnano.2009.379 pmid: 20023645 |
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
doi: 10.1038/nnano.2010.213 pmid: 21076404 |
| [38] |
doi: 10.1016/j.plrev.2012.05.010 pmid: 22658507 |
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
pmid: 8127706 |
| [49] |
|
| [50] |
COMSOL Multiphysics® v. 6.2. www.comsol.com. COMSOL AB, Stockholm, Sweden.
|
| [51] |
|
| [52] |
doi: 10.1021/nn5025829 pmid: 24933128 |
| [53] |
|
| [1] | Xu Yang, Zeying Zhang, Meng Su, Yanlin Song. Research Progress on Nano Photonics Technology-based SARS-CoV-2 Detection※ [J]. Acta Chimica Sinica, 2022, 80(1): 80-88. |
| [2] | Rongnan Yi, Yan Wu. Research Progress on Surface-Enhanced Raman Spectroscopy Technique for the Detection of microRNA [J]. Acta Chimica Sinica, 2021, 79(6): 694-704. |
| [3] | Lin Yao, Ying Yilun, Gao Rui, Wang Huifeng, Long Yitao. Analysis of Single-entity Anisotropy with a Solid-state Nanopore [J]. Acta Chim. Sinica, 2017, 75(7): 675-678. |
| [4] | Sha Jingjie, Xu Bing, Chen Yunfei, Yang Yanjing. Experimental Research of Protein Translocation Using Solid-state Nanopore [J]. Acta Chim. Sinica, 2017, 75(11): 1121-1125. |
| [5] | Hu Zhengli, Du Jihui, Ying Yilun, Peng Yueyi, Cao Chan, Long Yi-Tao. Single-Molecule Analysis of Colorectal Cancer-associated MicroRNAs via a Biological Nanopore [J]. Acta Chim. Sinica, 2017, 75(11): 1087-1090. |
| [6] | Li Xiaoli, Wang Yucong, Zhang Xuejing, Zhao Yunjie, Liu Chenghui, Li Zhengping. Double Strand-Specific Nuclease-Assisted Sensitive Detection of MicroRNA [J]. Acta Chimica Sinica, 2014, 72(3): 395-400. |
| [7] | Zhang Liang, Yu Miao, He Chuan. Mouse Tet1 Protein can Oxidize 5mC to 5hmC and 5caC on Single-stranded DNA [J]. Acta Chimica Sinica, 2012, 70(20): 2123-2126. |
| [8] | MENG Xiang-Xian, YANG Xiao-Hai, WANG Ke-Min, GUO Qiu-Beng, HUANG Jing-Shan. Gold Nanoparticle-Based Asymmetric PCR for Single-Strand DNA [J]. Acta Chimica Sinica, 2010, 68(9): 917-920. |
| [9] | BO Yang, YANG Qing, MENG Qi, HU Ying, HUANG Sha-Sheng. A Novel DNA Biosensor Modified with Hollow Gold Nanoparticles [J]. Acta Chimica Sinica, 2010, 68(07): 672-678. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||