Acta Chimica Sinica ›› 2019, Vol. 77 ›› Issue (9): 884-888.DOI: 10.6023/A19060220 Previous Articles Next Articles
Special Issue: 有机自由基化学; 纪念南开大学化学学科创建100周年
Communication
投稿日期:2019-06-18
发布日期:2019-08-13
通讯作者:
陈弓,何刚
E-mail:gongchen@nankai.edu.cn;hegang@nankai.edu.cn
基金资助:
Zhang, Heng, Mou, Xueqing, Chen, Gong*( ), He, Gang*(
), He, Gang*( )
)
Received:2019-06-18
Published:2019-08-13
Contact:
Chen, Gong,He, Gang
E-mail:gongchen@nankai.edu.cn;hegang@nankai.edu.cn
Supported by:Share
Zhang, Heng, Mou, Xueqing, Chen, Gong, He, Gang. Copper-catalyzed Intramolecular Aminoperfluoroalkylation Reaction of O-Homoallyl Benzimidates[J]. Acta Chimica Sinica, 2019, 77(9): 884-888.
| Entry | Catalyst | Additive | Solvent | Yielda/% of 2 |
|---|---|---|---|---|
| 1b | Cu(CH3CN)4PF6 | BOX ligand | DCE | NR |
| 2 | Cu(CH3CN)4PF6 | AgOAc | DCE | 44 |
| 3 | CuCl | AgOAc | DCE | 54 |
| 4 | CuBr | AgOAc | DCE | 62 |
| 5 | CuI | AgOAc | DCE | 60 |
| 6 | Cu(acac)2 | AgOAc | DCE | 54 |
| 7 | Cu(OTf)2 | AgOAc | DCE | 45 |
| 8 | Cu(OAc)2 | AgOAc | DCE | 72 (64)c |
| 9 | Cu(OAc)2 | AgOTFA | DCE | 19 |
| 10 | Cu(OAc)2 | AgOTf | DCE | 47 |
| 11 | Cu(OAc)2 | Ag2CO3 | DCE | 53 |
| 12 | Cu(OAc)2 | Cs2CO3 | DCE | 20 |
| 13 | Cu(OAc)2 | AgOAc | CH3CN | 67 |
| 14 | Cu(OAc)2 | AgOAc | THF | 52 |
| 15 | Cu(OAc)2 | AgOAc | EtOAc | 63 |
| 16 | Cu(OAc)2 | — | DCE | NR |
| 17 | AgOAc | DCE | <5 | |
| 18d | Cu(OAc)2 | AgOAc | DCE | <5 |
| 19e | Cu(OAc)2 | AgOAc | DCE | 53 |
| Entry | Catalyst | Additive | Solvent | Yielda/% of 2 |
|---|---|---|---|---|
| 1b | Cu(CH3CN)4PF6 | BOX ligand | DCE | NR |
| 2 | Cu(CH3CN)4PF6 | AgOAc | DCE | 44 |
| 3 | CuCl | AgOAc | DCE | 54 |
| 4 | CuBr | AgOAc | DCE | 62 |
| 5 | CuI | AgOAc | DCE | 60 |
| 6 | Cu(acac)2 | AgOAc | DCE | 54 |
| 7 | Cu(OTf)2 | AgOAc | DCE | 45 |
| 8 | Cu(OAc)2 | AgOAc | DCE | 72 (64)c |
| 9 | Cu(OAc)2 | AgOTFA | DCE | 19 |
| 10 | Cu(OAc)2 | AgOTf | DCE | 47 |
| 11 | Cu(OAc)2 | Ag2CO3 | DCE | 53 |
| 12 | Cu(OAc)2 | Cs2CO3 | DCE | 20 |
| 13 | Cu(OAc)2 | AgOAc | CH3CN | 67 |
| 14 | Cu(OAc)2 | AgOAc | THF | 52 |
| 15 | Cu(OAc)2 | AgOAc | EtOAc | 63 |
| 16 | Cu(OAc)2 | — | DCE | NR |
| 17 | AgOAc | DCE | <5 | |
| 18d | Cu(OAc)2 | AgOAc | DCE | <5 |
| 19e | Cu(OAc)2 | AgOAc | DCE | 53 |
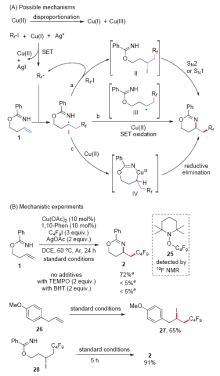
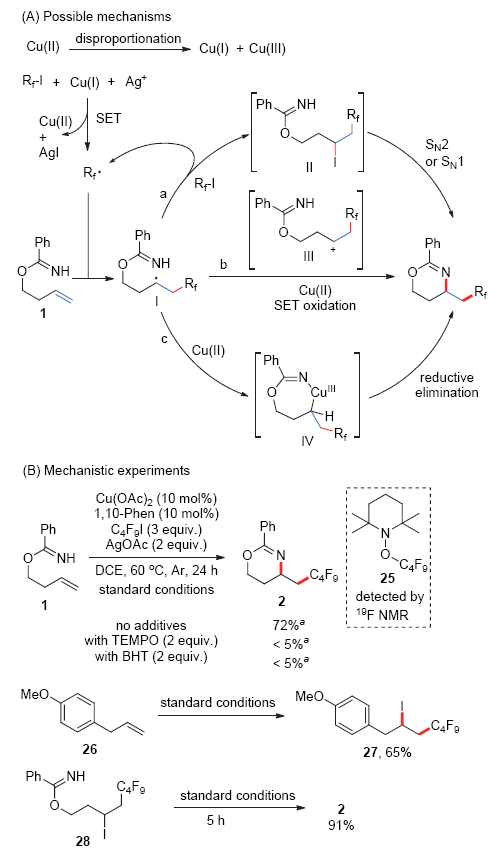
| [1] |
Hu, J.; Ding, K . Acta Chim. Sinica 2018, 76, 905 (in Chinese).
doi: 10.6023/A1812E001 |
|
( 胡金波, 丁奎岭, 化学学报 , 2018, 76, 905.)
doi: 10.6023/A1812E001 |
|
| [2] |
(a) Smart, B. E . J. Flurorine Chem. 2001, 109, 3
doi: 10.1021/jm800219f |
|
(b) Hagmann, W. K . J. Med. Chem. 2008, 51, 4359;
doi: 10.1021/jm800219f |
|
|
(c) Purser, S.; Moore, P. R.; Swallow, S.; Gouverneur, V. Chem. Soc. Rev. 2008, 37, 320;
doi: 10.1021/jm800219f |
|
|
(d) Zhou, Y.; Wang, J.; Gu, Z.; Wang, S.; Zhu, W.; Acena, J. L.; Soloshonok, V. A.; Izawa, K.; Liu, H. Chem. Rev. 2016, 116, 422.
doi: 10.1021/jm800219f |
|
| [3] |
(a) Vitaku, E.; Smith, D. T.; Njardarson, J. T. J. Med. Chem. 2014, 57, 10257
doi: 10.1021/jm501100b |
|
(b) Meyer, F. Chem. Commun. 2016, 52, 3077.
doi: 10.1021/jm501100b |
|
| [4] |
Tian, Y.; Chen, S.; Gu, Q.-S.; Lin, J.-S.; Liu, X.-Y . Tetrahedron Lett. 2018, 59, 203.
doi: 10.1016/j.tetlet.2017.12.034 |
| [5] |
(a) Takamasa, F.; Yoshiko, S.; Hisao, U . Chem. Lett. 1987, 16, 521
doi: 10.1246/cl.1987.521 |
|
(b) Kim, E.; Choi, S.; Kim, H.; Cho, E. J. Chem.-Eur. J. 2013, 19, 6209;
doi: 10.1246/cl.1987.521 |
|
|
(c) Matcha, K.; Antonchick, A. P. Angew. Chem., Int. Ed. 2014, 53, 11960;
doi: 10.1246/cl.1987.521 |
|
|
(d) Wei, Q.; Chen, J.-R.; Hu, X.-Q.; Yang, X.-C.; Lu, B.; Xiao, W.-J. Org. Lett. 2015, 17, 4464;
doi: 10.1246/cl.1987.521 |
|
|
(e) Jarrige, L.; Carboni, A.; Dagousset, G.; Levitre, G.; Magnier, E.; Masson, G. Org. Lett. 2016, 18, 2906.
doi: 10.1246/cl.1987.521 |
|
| [6] |
For copper catalyzed intramolecular aminoperfuoroalkylation, see: (a) Egami, H.; Kawamura, S.; Miyazaki, A.; Sodeoka, M. Angew. Chem., Int. Ed. 2013, 52, 7841
doi: 10.1002/anie.v52.30 |
|
(b) Kawamura, S.; Egami, H.; Sodeoka, M. J. Am. Chem. Soc. 2015, 137, 4865;
doi: 10.1002/anie.v52.30 |
|
|
(c) Kawamura, S.; Dosei, K.; Valverde, E.; Ushida, K.; Sodeoka, M . J. Org. Chem. 2017, 82, 12539;
doi: 10.1002/anie.v52.30 |
|
|
(d) Lin, J.-S.; Liu, X.-G.; Zhu, X.-L.; Tan, B.; Liu, X.-Y . J. Org. Chem. 2014, 79, 7084;
doi: 10.1002/anie.v52.30 |
|
|
(e) Lin, J.-S.; Xiong, Y.-P.; Ma, C.-L.; Zhao, L.-J.; Tan, B.; Liu, X.-Y . Chem.-Eur. J. 2014, 20, 1332;
doi: 10.1002/anie.v52.30 |
|
|
(f) Li, X.-F.; Lin, J.-S.; Liu, X.-Y . Synthesis 2017, 49, 4213;
doi: 10.1002/anie.v52.30 |
|
|
(g) Shen, K.; Wang, Q . Org. Chem. Front. 2016, 3, 222;
doi: 10.1002/anie.v52.30 |
|
|
(h) Yu, L.-Z.; Wei, Y.; Shi, M . Chem. Commun. 2016, 52, 13163;
doi: 10.1002/anie.v52.30 |
|
|
(i) Zhang, H.-Y.; Huo, W.; Ge, C.; Zhao, J.; Zhang, Y . Synlett 2017, 28, 962;
doi: 10.1002/anie.v52.30 |
|
|
(j) Chang, B.; Su, Y.; Huang, D.; Wang, K.-H.; Zhang, W.; Shi, Y.; Zhang, X.; Hu, Y . J. Org. Chem. 2018, 83, 4365.
doi: 10.1002/anie.v52.30 |
|
| [7] |
For enantioselective aminotrifluoromethylation of alkene, see: (a) Lin, J.-S.; Dong, X.-Y.; Li, T.-T.; Jiang, N.-C.; Tan, B.; Liu, X.-Y. J. Am. Chem. Soc. 2016, 138, 9357
doi: 10.1021/jacs.6b04077 |
|
(b) Lin, J.-S.; Wang, F.-L.; Dong, X.-Y.; He, W.-W.; Yuan, Y.; Chen, S.; Liu, X.-Y . Nat. Commun. 2017, 8, 14841.
doi: 10.1021/jacs.6b04077 |
|
| [8] |
For recent examples of using imidates as nucleophile, see: (a) Brindle, C. S.; Yeung, C. S.; Jacobsen, E. N. Chem. Sci. 2013, 4, 2100
doi: 10.1039/c3sc50410g |
|
(b) Zhu, R.; Yu, K.; Gu, Z. Org. Biomol. Chem. 2014, 12, 6653.
doi: 10.1039/c3sc50410g |
|
| [9] |
Mou, X.-Q.; Chen, X.-Y.; Chen, G.; He, G . Chem. Commun. 2018, 54, 515.
doi: 10.1039/C7CC08897C |
| [10] |
For selected examples of intramolecular C-H amination of imidates by other groups, see: (a) Wappes, E. A.; Nakafuku, K. M.; Nagib, D. A. J. Am. Chem. Soc. 2017, 139, 10204
doi: 10.1021/jacs.7b05214 |
|
(b) Stateman, L. M.; Wappes, E. A.; Nakafuku, K. M.; Edwards, K. M.; Nagib, D. A. Chem. Sci. 2019, 10, 2693;
doi: 10.1021/jacs.7b05214 |
|
|
(c) Shaw, M.; Kumar, A. Org. Lett. 2019, 21, 3108.
doi: 10.1021/jacs.7b05214 |
|
| [11] |
Mou, X.-Q.; Rong, F.-M.; Zhang, H.; Chen, G.; He, G . Org. Lett. 2019, 21, 4657.
doi: 10.1021/acs.orglett.9b01552 |
| [12] |
(a) Eisenberger, P.; Gischig, S.; Togni, A . Chem.-Eur. J. 2006, 12, 2579
doi: 10.1002/(ISSN)1521-3765 |
|
(b) Matoušek, V.; Pietrasiak, E.; Schwenk, R.; Togni, A. J. Org. Chem. 2013, 78, 6763;
doi: 10.1002/(ISSN)1521-3765 |
|
|
(c) Charpentier, J.; Früh, N.; Togni, A . Chem. Rev. 2015, 115, 650.
doi: 10.1002/(ISSN)1521-3765 |
|
| [13] |
For selected reviews on the synthesis and application of perfluoroalkyl iodides, see: (a) Huang, B . Chin. J. Org. Chem. 1981, 1, 403 (in Chinese).
doi: 10.6023/cjoc201808030 |
|
( 黄炳南 , 有机化学, 1981, 1, 403.);
doi: 10.6023/cjoc201808030 |
|
|
(b) Brace, N. O . J. Flurorine Chem. 1999, 93, 1;
doi: 10.6023/cjoc201808030 |
|
|
(c) Brace, N. O . J. Flurorine Chem. 1999, 96, 101;
doi: 10.6023/cjoc201808030 |
|
|
(d) Brace, N. O . J. Flurorine Chem. 2001, 108, 147;
doi: 10.6023/cjoc201808030 |
|
|
(e) Murphy, P. M.; Baldwin, C. S.; Buck, R. C . J. Flurorine Chem. 2012, 138, 3;
doi: 10.6023/cjoc201808030 |
|
|
(f) Huang, H.; Wang, X.; Wang, J . Chin. J. Org. Chem. 2019, 39, 1 (in Chinese).
doi: 10.6023/cjoc201808030 |
|
|
( 黄航, 王兮, 王剑波, 有机化学, 2019, 39, 1.)
doi: 10.6023/cjoc201808030 |
|
| [14] |
(a) Chen, Q.-Y.; Chen, Y.-X.; Huang, W.-Y. Acta Chim. Sinica 1984, 42, 906 (in Chinese).
doi: 10.1002/cjoc.v31.7 |
|
( 陈庆云, 陈亚雄, 黄维垣, 化学学报 , 1984, 42, 906.);
doi: 10.1002/cjoc.v31.7 |
|
|
(b) Chen, Q.-Y.; Yang, Z.-Y . J. Flurorine Chem. 1985, 28, 399;
doi: 10.1002/cjoc.v31.7 |
|
|
(c) Chen, Q.-Y.; Yang, Z.-Y . Acta Chim. Sinica 1985, 43, 1073 (in Chinese).
doi: 10.1002/cjoc.v31.7 |
|
|
(陈庆云, 杨震宇, 化学学报, 1985, 43, 1073.);
doi: 10.1002/cjoc.v31.7 |
|
|
(d) Chen, Q.-Y.; Yang, Z.-Y . Acta Chim. Sinica 1985, 43, 1118 (in Chinese).
doi: 10.1002/cjoc.v31.7 |
|
|
(陈庆云, 杨震宇, 化学学报, 1985, 43, 1118.);
doi: 10.1002/cjoc.v31.7 |
|
|
(e) Chen, Q.-Y.; Yang, Z.-Y . Acta Chim. Sinica 1986, 44, 265 (in Chinese).
doi: 10.1002/cjoc.v31.7 |
|
|
(陈庆云, 杨震宇, 化学学报, 1986, 44, 265.);
doi: 10.1002/cjoc.v31.7 |
|
|
(f) Chen, Q.-Y.; Yang, Z.-Y . Acta Chim. Sinica 1986, 44, 1025 (in Chinese).
doi: 10.1002/cjoc.v31.7 |
|
|
(陈庆云, 杨震宇, 化学学报, 1986, 44, 1025.);
doi: 10.1002/cjoc.v31.7 |
|
|
(g) Chen, Q.-Y.; Qiu, Z.-M . Acta Chim. Sinica 1987, 45, 354 (in Chinese).
doi: 10.1002/cjoc.v31.7 |
|
|
(陈庆云, 裘再明, 化学学报, 1987, 45, 354.);
doi: 10.1002/cjoc.v31.7 |
|
|
(h) Chen, Q.-Y.; Qiu, Z.-M . Acta Chim. Sinica 1988, 46, 258 (in Chinese).
doi: 10.1002/cjoc.v31.7 |
|
|
(陈庆云, 裘再明, 化学学报, 1988, 46, 258.);
doi: 10.1002/cjoc.v31.7 |
|
|
(i) Chen, Q.-Y.; Chen, J.-G . Acta Chim. Sinica 1988, 46, 301 (in Chinese).
doi: 10.1002/cjoc.v31.7 |
|
|
(陈庆云, 杨建国, 化学学报, 1988, 46, 301.);
doi: 10.1002/cjoc.v31.7 |
|
|
(j) Xiao, Z.; Hu, H.; Ma, J.; Chen, Q.; Guo, Y . Chin. J. Chem. 2013, 31, 939;
doi: 10.1002/cjoc.v31.7 |
|
|
(k) Su, Z.; Guo, Y.; Chen, Q.-Y.; Zhao, Z.-G.; Nian, B-Y . Chin. J. Chem. 2019, 37, 597.
doi: 10.1002/cjoc.v31.7 |
|
| [15] |
For selected examples of perfluoroalkylation of aromatic compounds with perfluoroalkyl iodides, see (a) Iqbal, N.; Choi, S.; Ko, E.; Cho, E. J. Tetrahedron Lett. 2012, 53, 2005
doi: 10.1016/j.tetlet.2012.02.032 |
|
(b) Barata-Vallejo, S.; Flesia, M. M.; Lantaño, B.; Argüello, J. E.; Peñéñory, A. B.; Postigo, A. Eur. J. Org. Chem. 2013, 2013, 998;
doi: 10.1016/j.tetlet.2012.02.032 |
|
|
(c) Straathof, N. J. W.; Gemoets, H. P. L.; Wang, X.; Schouten, J. C.; Hessel, V.; Noël, T . ChemSusChem 2014, 7, 1612;
doi: 10.1016/j.tetlet.2012.02.032 |
|
|
(d) Huang, Y.; Lei, Y.-Y.; Zhao, L.; Gu, J.; Yao, Q.; Wang, Z.; Li, X.-F.; Zhang, X.; He, C.-Y . Chem. Commun. 2018, 54, 13662;
doi: 10.1016/j.tetlet.2012.02.032 |
|
|
(e) Yerien, D. E.; Cooke, M. V.; García Vior, M. C.; Barata- Vallejo, S.; Postigo, A . Org. Biomol. Chem. 2019, 17, 3741.
doi: 10.1016/j.tetlet.2012.02.032 |
|
| [16] |
For selected examples of perfluoroalkylation of alkene with perfluoroalkyl iodides under visible light irradiation, see (a) Brace, N. O. J. Org. Chem. 1963, 28, 3093
doi: 10.1021/jo01046a039 |
|
(b) Habib, M. H.; Mallouk, T. E. J. Flurorine Chem. 1991, 53, 53;
doi: 10.1021/jo01046a039 |
|
|
(c) Ogawa, A.; Imura, M.; Kamada, N.; Hirao, T . Tetrahedron Lett. 2001, 42, 2489;
doi: 10.1021/jo01046a039 |
|
|
(d) Tsuchii, K.; Imura, M.; Kamada, N.; Hirao, T.; Ogawa, A . J. Org. Chem. 2004, 69, 6658;
doi: 10.1021/jo01046a039 |
|
|
(e) Wallentin, C.-J.; Nguyen, J. D.; Finkbeiner, P.; Stephenson, C. R. J . J. Am. Chem. Soc. 2012, 134, 8875;
doi: 10.1021/jo01046a039 |
|
|
(f) Mizuta, S.; Verhoog, S.; Engle, K. M.; Khotavivattana, T.; O’Duill, M.; Wheelhouse, K.; Rassias, G.; Médebielle, M.; Gouverneur, V . J. Am. Chem. Soc. 2013, 135, 2505;
doi: 10.1021/jo01046a039 |
|
|
(g) Wang, Y.; Wang, J.; Li, G.-X.; He, G.; Chen, G . Org. Lett. 2017, 19, 1442;
doi: 10.1021/jo01046a039 |
|
|
(h) Beniazza, R.; Remisse, L.; Jardel, D.; Lastécouères, D.; Vincent, J.-M . Chem. Commun. 2018, 54, 7451;
doi: 10.1021/jo01046a039 |
|
|
(j) Rawner, T.; Lutsker, E.; Kaiser, C. A.; Reiser, O . ACS Catal. 2018, 8, 3950.
doi: 10.1021/jo01046a039 |
|
| [17] |
For selected examples of transition metal catalyzed perfluoroalkylation of alkene with perfluoroalkyl iodides, see: (a) Gil-Rubio, J.; Guerrero-Leal, J.; Blaya, M.; Vicente, J.; Bautista, D.; Jones, P. G . Organometallics 2012, 31, 1287
doi: 10.1021/om2009588 |
|
(b) Blaya, M.; Bautista, D.; Gil- Rubio, J.; Vicente, J . Organometallics 2017, 36, 1245;
doi: 10.1021/om2009588 |
|
|
(c) Zheng, J.; Chen, P.; Yuan, Y.; Cheng, J . J. Org. Chem. 2017, 82, 5790.
doi: 10.1021/om2009588 |
|
| [18] |
For selected reviews on the synthesis and application of 1, 3-oxazines, see: (a) Schmidt, R. R .Synthesis 1972, 1972, 333
doi: 10.1055/s-1972-21882 |
|
(b) Sato, M.; Sunami, S.; Kaneko, C . Heterocycles 1996, 42, 861.
doi: 10.1055/s-1972-21882 |
|
| [19] | In the reaction of O-homoallyl benzimidates equipped with multi- substituted alkene and trichloroacetimidate analogue of 1, no desired product was detected, and most of the starting material remain unconsumed. |
| [1] | Zhanglong Yu, Zhongliang Li, Changjiang Yang, Qiangshuai Gu, Xinyuan Liu. Research Progress on Copper-Catalyzed Enantioselective Desymmetrization of Diols★ [J]. Acta Chimica Sinica, 2023, 81(8): 955-966. |
| [2] | Kongxi Qiu, Jie Li, Haowen Ma, Wei Zhou, Qian Cai. Recent Advances in the Construction of Nitrogen-Containing Heterocycles via Trapping Organocopper(I) Intermediates [J]. Acta Chimica Sinica, 2023, 81(1): 42-63. |
| [3] | Qinghao Xu, Lipu Wei, Zhen Zhang, Bin Xiao. Copper Promoted Synthesis of Tetraalkylgermanes from Germanium Electrophiles and Alkyl Bromides※ [J]. Acta Chimica Sinica, 2022, 80(4): 428-431. |
| [4] | Zhang Ronghua, Xu Bing, Zhang Zhanming, Zhang Junliang. Ming-Phos/Copper(I)-Catalyzed Asymmetric[3+2] Cycloaddition of Azomethine Ylides with Nitroalkenes [J]. Acta Chimica Sinica, 2020, 78(3): 245-249. |
| [5] | Liang Huan, Gou Along, Gao Zhupeng, Lei Linsheng, Wang Bowen, Yu Lan, Xu Xuetao, Wang Shaohua. A New Strategy for the Synthesis of Tertiary Amides via a Copper-Catalyzed Decyanation Reaction of N,N-Disubstituted 2-Aminomalononitriles [J]. Acta Chimica Sinica, 2020, 78(10): 1064-1068. |
| [6] | Lin, Fengguirong, Liang, Yujie, Li, Xinyao, Song, Song, Jiao, Ning. Copper-catalyzed ortho C-H Azidation of Anilines Using Molecular Oxygen as Terminal Oxidant [J]. Acta Chimica Sinica, 2019, 77(9): 906-910. |
| [7] | Cheng, Zhongming, Chen, Pinhong, Liu, Guosheng. Enantioselective Cyanation of Remote C—H Bonds via Cooperative Photoredox and Copper Catalysis [J]. Acta Chimica Sinica, 2019, 77(9): 856-860. |
| [8] | Li Xue-Fei, Lin Jin-Shun, Wang Jian, Li Zhong-Liang, Gu Qiang-Shuai, Liu Xin-Yuan. Cu/Chiral Phosphoric Acid-Catalyzed Asymmetric Radical-Initiated Aminoarylation of Alkenes [J]. Acta Chimica Sinica, 2018, 76(11): 878-882. |
| [9] | Li Xin-Ling, Wang Jia-Qi, Li Long, Yin Ying-Wu, Ye Long-Wu. Facile Synthesis of 2H-Pyrroles: Combination of Gold Catalysis and Lewis Acid Catalysis [J]. Acta Chim. Sinica, 2016, 74(1): 49-53. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||