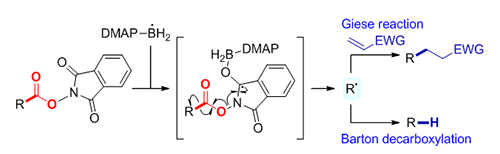
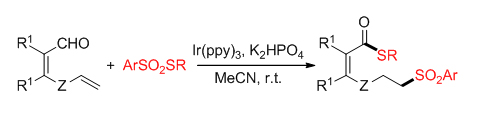
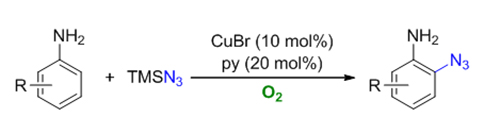
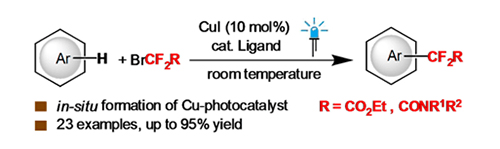
-
-
 About the Cover:
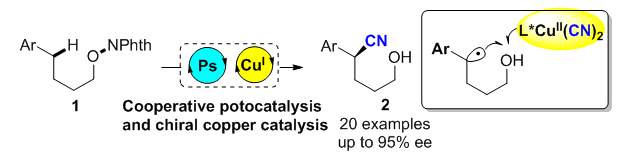
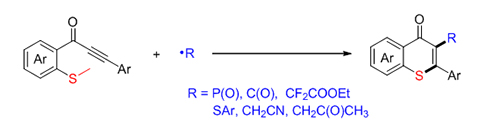
About the Cover:On the cover: Cooperating photo-redox catalysis with copper catalysis via the Radical Relay Process can achieve the directed functionalization of the inactive Csp3—H bond, consequently providing an efficient enantioselective approach to the direct construction of the remote function groups through the chiral ligand regulation. [Liu, Guosheng et al. on page 856-860.]
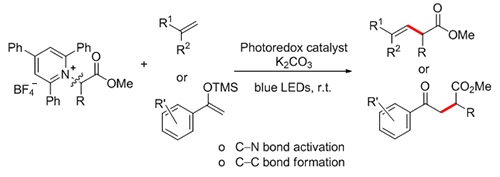
On the back cover: On the basis of visible light-excited Ir-catalyzed radical approach, two nitrogen-containing species, aromatic tertiary amines and nitrones can couple at the N-α positions to give β-amino hydroxylamines, which could be readily converted into vicinal diamines. The mechanism of this reaction involves an efficient umpolung of aromatic tertiary amines to generate α-aminoalkyl radicals through photocatalytic single electron oxidation and α-deprotonation. [Huang, Pei-Qiang et al. on page 850-855.]
Special Issues:Organic Free Radical Chemistry
2019, 77(9)
15 September 2019
-
-
Current Issue
Perspective
Review
Communication
Article