Acta Chimica Sinica ›› 2021, Vol. 79 ›› Issue (11): 1394-1400.DOI: 10.6023/A21080358 Previous Articles Next Articles
Article
投稿日期:2021-08-01
发布日期:2021-09-10
通讯作者:
薛小松
基金资助:
Danqi Zhang, Yingbo Shao, Hanliang Zheng, Biying Zhou, Xiao-Song Xue( )
)
Received:2021-08-01
Published:2021-09-10
Contact:
Xiao-Song Xue
Supported by:Share
Danqi Zhang, Yingbo Shao, Hanliang Zheng, Biying Zhou, Xiao-Song Xue. Mechanistic Study on the Bidentate Nitrogen-Ligated Iodine(V) Reagent Promoted Oxidative Dearomatization of Phenols[J]. Acta Chimica Sinica, 2021, 79(11): 1394-1400.
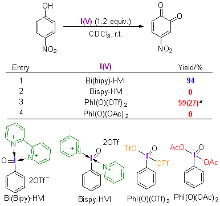
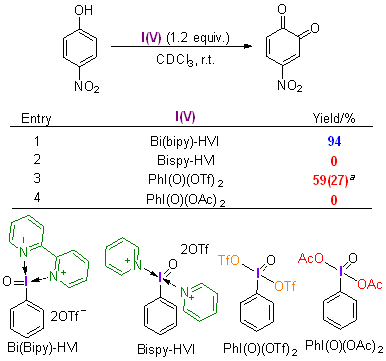
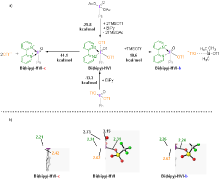
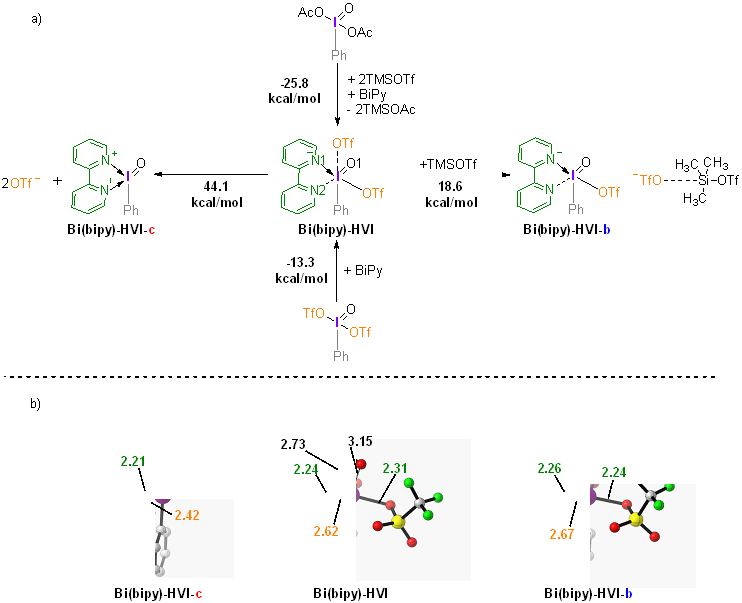
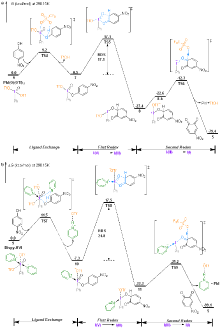
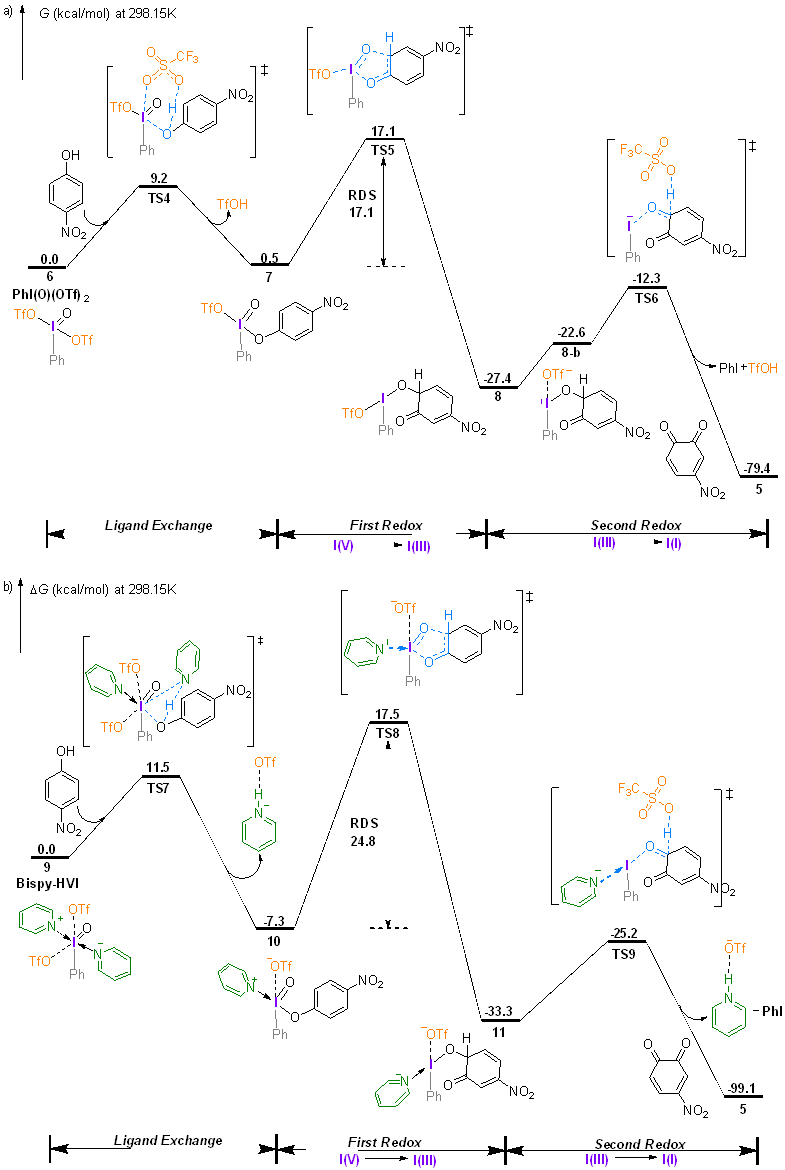
| [1] |
(a) Zhdankin V. V. Hypervalent Iodine Chemistry: Preparation, Structure, and Synthetic Applications of Polyvalent Iodine Compounds, Wiley, New York, 2013.
pmid: 26861673 |
|
(b) Chen J.; Qu H.; Peng J.; Chen C. Chin. J. Org. Chem. 2015, 35, 937. (in Chinese)
doi: 10.6023/cjoc201501004 pmid: 26861673 |
|
|
( 陈静, 曲红梅, 彭静, 陈超, 有机化学, 2015, 35, 937.)
doi: 10.6023/cjoc201501004 pmid: 26861673 |
|
|
(c) Duan Y.; Jiang S.; Han Y.; Sun B.; Zhang C. Chin. J. Org. Chem. 2016, 36, 1973. (in Chinese)
doi: 10.6023/cjoc201605007 pmid: 26861673 |
|
|
( 段亚南, 姜山, 韩永超, 孙博, 张弛, 有机化学, 2016, 36, 1973.)
doi: 10.6023/cjoc201605007 pmid: 26861673 |
|
|
(d) Yoshimura A.; Zhdankin V. V. Chem. Rev. 2016, 116, 3328.
doi: 10.1021/acs.chemrev.5b00547 pmid: 26861673 |
|
|
(e) Zhang X.; Cong Y.; Lin G.; Guo X.; Cao Y.; Lei K.; Du Y. Chin. J. Org. Chem. 2016, 36, 2513. (in Chinese)
doi: 10.6023/cjoc201605034 pmid: 26861673 |
|
|
( 张翔, 丛颖, 林光宇, 郭旭亮, 曹阳, 雷坤华, 杜云飞, 有机化学, 2016, 36, 2513.)
doi: 10.6023/cjoc201605034 pmid: 26861673 |
|
|
(f) Ma J.; Chen L.; Yuan Z.; Cheng H. Chin. J. Org. Chem. 2018, 38, 1586. (in Chinese)
doi: 10.6023/cjoc201802021 pmid: 26861673 |
|
|
( 马姣丽, 陈立成, 袁中文, 程辉成, 有机化学, 2018, 38, 1586.)
doi: 10.6023/cjoc201802021 pmid: 26861673 |
|
|
(g) Parra A. Chem. Rev. 2019, 119, 12033.
doi: 10.1021/acs.chemrev.9b00338 pmid: 26861673 |
|
|
(h) Cai Q.; Ma H. Acta Chim. Sinica 2019, 77, 213. (in Chinese)
doi: 10.6023/A18110470 pmid: 26861673 |
|
|
( 蔡倩, 马浩文, 化学学报, 2019, 77, 213.)
doi: 10.6023/A18110470 pmid: 26861673 |
|
|
(i) Zhang B.; Li X.; Guo B.; Du Y. Chem. Commun. 2020, 56, 14119.
doi: 10.1039/D0CC05354F pmid: 26861673 |
|
|
(j) Yang X.; Hu Z.; Jia M.; Du F.; Zhang C. Synlett 2021, 32, 1289.
doi: 10.1055/a-1492-4943 pmid: 26861673 |
|
| [2] |
(a) Qin K.; Su G.; Rao W.; Tan G. M. Chin. J. Org. Chem. 2006, 26, 1623. (in Chinese)
|
|
( 覃开云, 苏桂发, 饶万平, 谭光明, 有机化学, 2006, 26, 1623.)
|
|
|
(b) Satam V.; Harad A.; Rajule R.; Pati H. Tetrahedron 2010, 66, 7659.
doi: 10.1016/j.tet.2010.07.014 |
|
|
(c) Duschek A.; Kirsch S. F. Angew. Chem. Int. Ed. 2011, 50, 1524.
doi: 10.1002/anie.201000873 |
|
|
(d) Zhang S.; Wu H.; Tang Y. Chin. J. Org. Chem. 2021, 41, 490. (in Chinese)
doi: 10.6023/cjoc202007030 |
|
|
( 张书瑜, 吴昊天, 汤峨, 有机化学, 2021, 41, 490.)
doi: 10.6023/cjoc202007030 |
|
| [3] |
(a) Pierpont C. G. Coord. Chem. Rev. 2001, 216, 99.
|
|
(b) Kharisov B. I.; Méndez-Rojas M. A.; Garnovskii A. D.; Ivakhnenko E. P.; Ortiz-Méndez U. J. Coord. Chem. 2002, 55, 745.
doi: 10.1080/0095897022000001511 |
|
|
(c) Sun L.; Campbell M. G.; Dinca M. Angew. Chem. Int. Ed. 2016, 55, 3566.
doi: 10.1002/anie.201506219 |
|
|
(d) Sato S.; Sakata K.; Hashimoto Y.; Takikawa H.; Suzuki K. Angew. Chem. Int. Ed. 2017, 56, 12608.
doi: 10.1002/anie.v56.41 |
|
|
(e) Esguerra K. V. N.; Lumb J.-P. Angew. Chem. Int. Ed. 2018, 57, 1514.
doi: 10.1002/anie.v57.6 |
|
|
(f) Zhang R.; Luo S. Chin. Chem. Lett. 2018, 29, 1193.
doi: 10.1016/j.cclet.2018.02.009 |
|
| [4] |
(a) Magdziak D.; Rodriguez A. A.; Van De Water R. W.; Pettus T. R. Org. Lett. 2002, 4, 285.
pmid: 29236486 |
|
(b) Lebrasseur N.; Gagnepain J.; Ozanne-Beaudenon A.; Léger J. M.; Quideau S. J. Org. Chem. 2007, 72, 6280.
pmid: 29236486 |
|
|
(c) Wu A.; Duan Y.; Xu D.; Penning T. M.; Harvey R. G. Tetrahedron 2010, 66, 2111.
doi: 10.1016/j.tet.2009.12.022 pmid: 29236486 |
|
|
(d) Uyanik M., Mutsuga T., Ishihara K. Molecules 2012, 17, 8604.
doi: 10.3390/molecules17078604 pmid: 29236486 |
|
|
(e) Usui K.; Yamamoto K.; Shimizu T.; Okazumi M.; Mei B.; Demizu Y.; Kurihara M.; Suemune H. J. Org. Chem. 2015, 80, 6502.
doi: 10.1021/acs.joc.5b00759 pmid: 29236486 |
|
|
(f) Mishra A. K.; Moorthy J. N. J. Org. Chem. 2016, 81, 6472.
doi: 10.1021/acs.joc.6b01105 pmid: 29236486 |
|
|
(g) Uyanik M.; Mutsuga T.; Ishihara K. Angew. Chem. Int. Ed. 2017, 56, 3956.
doi: 10.1002/anie.201612463 pmid: 29236486 |
|
|
(h) Pulvirenti L.; Muccilli V.; Cardullo N.; Spatafora C.; Tringali C. J. Nat. Prod. 2017, 80, 1648.
doi: 10.1021/acs.jnatprod.7b00250 pmid: 29236486 |
|
|
(i) Bizzarri B. M.; Botta L.; Capecchi E.; Celestino I.; Checconi P.; Palamara A. T.; Nencioni L.; Saladino R. J. Nat. Prod. 2017, 80, 3247.
doi: 10.1021/acs.jnatprod.7b00665 pmid: 29236486 |
|
|
(j) Stack D. E.; Mahmud B. Synth. Commun. 2018, 48, 161.
doi: 10.1080/00397911.2017.1390586 pmid: 29236486 |
|
| [5] |
(a) Xiao X.; Greenwood N. S.; Wengryniuk S. E. Angew. Chem., Int. Ed. 2019, 131, 16327.
doi: 10.1002/ange.v131.45 |
|
(b) Xiao X.; Roth J. M.; Greenwood N. S.; Velopolcek M. K.; Aguirre J.; Jalali M.; Ariafard A.; Wengryniuk S. E. J. Org. Chem. 2021, 86, 6566.
doi: 10.1021/acs.joc.1c00375 |
|
| [6] |
(a) Weiss R.; Seubert J. Angew. Chem. Int. Ed. 1994, 33, 891.
doi: 10.1002/(ISSN)1521-3773 |
|
(b) Zhdankin V. V.; Koposov A. Y.; Yashin N. V. Tetrahedron Lett. 2002, 43, 5735.
doi: 10.1016/S0040-4039(02)01192-9 |
|
| [7] |
(a) Zhou B.; Yan T.; Xue, X. S.; Cheng, J. P. Org. Lett. 2016, 18, 6128.
doi: 10.1021/acs.orglett.6b03134 |
|
(b) Yan T.; Zhou, B.; Xue, X. S.; Cheng, J. P. J. Org. Chem. 2016, 81, 9006.
doi: 10.1021/acs.joc.6b01642 |
|
|
(c) Zhou B.; Xue X. S.; Cheng J. P. Tetrahedron Lett. 2017, 58, 1287.
doi: 10.1016/j.tetlet.2017.02.040 |
|
|
(d) Zhou B.; Haj M. K.; Jacobsen E. N.; Houk K. N.; Xue X. S. J. Am. Chem. Soc. 2018, 140, 15206.
doi: 10.1021/jacs.8b05935 |
|
|
(e) Zheng H.; Sang Y.; Houk K. N.; Xue X. S.; Cheng J. P. J. Am. Chem. Soc. 2019, 141, 16046.
doi: 10.1021/jacs.9b08243 |
|
|
(f) Yang J.; Li M.; Xue X. S. Chin. J. Chem. 2019, 37, 359.
doi: 10.1002/cjoc.v37.4 |
|
|
(g) Zheng H.; Xue X. S. Curr. Org. Chem. 2020, 4, 1.
|
|
| [8] |
Frisch M. J.; Trucks G. W.; Schlegel H. B.; Scuseria G. E.; Robb M. A.; Cheeseman J. R.; Scalmani G.; Barone V.; Petersson G. A.; Nakatsuji H.; Li X.; Caricato M.; Marenich A. V.; Bloino J.; Janesko B. G.; Gomperts R.; Mennucci B.; Hratchian H. P.; Ortiz J. V.; Izmaylov A. F.; Sonnenberg J. L.; Williams-Young D.; Ding F.; Lipparini F. Egidi F.; Goings J.; Peng B.; Petrone A.; Henderson T.; Ranasinghe D.; Zakrzewski V. G.; Gao J.; Rega N.; Zheng G.; Liang W.; Hada M.; Ehara M.; Toyota K.; Fukuda R.; Hasegawa J.; Ishida M.; Nakajima T.; Honda Y.; Kitao O.; Nakai H.; Vreven T.; Throssell K.; Montgomery Jr., J. A.; Peralta J. E.; Ogliaro F.; Bearpark M. J.; Heyd J. J.; Brothers E. N.; Kudin K. N.; Staroverov V. N.; Keith T. A.; Kobayashi R.; Normand J.; Raghavachari K.; Rendell A. P.; Burant J. C.; Iyengar S. S.; Tomasi J.; Cossi M.; Millam J. M.; Klene M.; Adamo C.; Cammi R.; Ochterski J. W.; Martin R. L.; Morokuma K.; Farkas O.; Foresman J. B.; Fox D. J. Gaussian 16, Revision A.03, Gaussian, Inc., Wallingford CT, 2016.
|
| [9] |
Marenich A. V.; Cramer C. J.; Truhlar D. G. J. Phys. Chem. B 2009, 113, 6378.
doi: 10.1021/jp810292n |
| [10] |
Zhao Y.; Truhlar D. G. Acc. Chem. Res. 2008, 41, 157.
doi: 10.1021/ar700111a |
| [11] |
Hay P. J.; Wadt W. R. J. Chem. Phys. 1985, 82, 299.
doi: 10.1063/1.448975 |
| [12] |
(a) Weigend F.; Furche F.; Ahlrichs R. J. Chem. Phys. 2003, 119, 12753.
doi: 10.1063/1.1627293 |
|
(b) Weigend F.; Ahlrichs R. Phys. Chem. Chem. Phys. 2005, 7, 3297.
doi: 10.1039/b508541a |
|
|
(c) Jiang H.; Sun T. Y.; Wang X.; Xie Y.; Zhang X.; Wu Y. D.; Schaefer III H. F. Org. Lett. 2017, 19, 6502.
doi: 10.1021/acs.orglett.7b03167 |
|
|
(d) Sun T.; Chen K.; Zhou H.; You T.; Yin P.; Wang X. J. Comput. Chem. 2021, 42, 470.
doi: 10.1002/jcc.v42.7 |
|
| [13] |
Legault C. Y. CYLview, 1.0b, Université de Sherbrooke, 2009;
|
| [14] |
The PyMOL Molecular Graphics System, Version 2.0.4, Schrödinger, LLC.
|
| [15] |
Kaur A.; Ariafard A. Org. Biomol. Chem. 2020, 18, 1117.
doi: 10.1039/C9OB02650A |
| [16] |
(a) Feldman K. S.; Sambandam A.; Bowers K. E.; Appel H. M. J. Org. Chem. 1999, 64, 5794.
doi: 10.1021/jo982477n pmid: 17226982 |
|
(b) Mitchell J. S.; Wu Y.; Cook C. J.; Main L. Bioconjugate Chem. 2007, 18, 268.
pmid: 17226982 |
|
| [17] |
在审稿期间,Ariafard等人在有机化学杂志发表了相关反应的机理研究工作, 该工作得到了与我们工作相似的结论: Jalali M.; Bissember A. C.; Yates B. F..; Wengryniuk S. E.; Ariafard A. J. Org. Chem. 2021, 86, 12237.
|
| [1] | Juan Wang, Huamin Xiao, Ding Xie, Yuanru Guo, Qingjiang Pan. Density Functional Theory Study of Structures of Copper-doped and Graphitic Carbon Nitride-combined Zinc Oxides and Their Boosted Nitrogen Dioxide-sensing Performance [J]. Acta Chimica Sinica, 2023, 81(11): 1493-1499. |
| [2] | Li Man, Kang Huiying, Xue Xiao-Song, Cheng Jin-Pei. Computational Study of the Trifluoromethyl Radical Donor Abilities of CF3 Sources [J]. Acta Chim. Sinica, 2018, 76(12): 988-996. |
| [3] | Xu Chenyu, Lin Jiayi, Pan Fuqiang, Deng Bowen, Wang Zhihua, Zhou Junhu, Chen Yun, Ma Jingcheng, Gu Zhien, Zhang Yanwei. Photo-thermochemical Cycle for CO2 Reduction based on Effective Ni ion Substitute-doped TiO2 [J]. Acta Chim. Sinica, 2017, 75(7): 699-707. |
| [4] | CHEN Qin-Wen, WANG Lan-Ying*1, ZHAI Gao-Hong, WEN Zhen-Yi2, ZHANG Zu-Xun. Study on Electronic Spectra of 2-Styryl-β-naphthathiazole Dyes with Time-dependent Density Functional Theory [J]. Acta Chimica Sinica, 2005, 63(1): 39-43. |
| [5] | WEI Yong-Qin, WU Ke-Chen, LIN Cheng-Sheng, MANG Chao-Yong, LIU Ping, ZHANG Ming-Xin, HONG Tao, ZHOU Zhang-Feng, ZHUANG Bo-Tao. Basisset Effects in the DFT Calculations of Hyperpolarizability [J]. Acta Chimica Sinica, 2004, 62(6): 578-582. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||