Acta Chimica Sinica ›› 2021, Vol. 79 ›› Issue (11): 1385-1393.DOI: 10.6023/A21060282 Previous Articles Next Articles
Article
赵添堃*( ), 王鹏, 姬明宇, 李善家, 杨明俊, 蒲秀瑛
), 王鹏, 姬明宇, 李善家, 杨明俊, 蒲秀瑛
投稿日期:2021-06-21
发布日期:2021-08-16
通讯作者:
赵添堃
基金资助:
Tiankun Zhao( ), Peng Wang, Mingyu Ji, Shanjia Li, Mingjun Yang, Xiuying Pu
), Peng Wang, Mingyu Ji, Shanjia Li, Mingjun Yang, Xiuying Pu
Received:2021-06-21
Published:2021-08-16
Contact:
Tiankun Zhao
Supported by:Share
Tiankun Zhao, Peng Wang, Mingyu Ji, Shanjia Li, Mingjun Yang, Xiuying Pu. Post-Synthetic Modification Research of Salan Titanium bis-Chelates via Sonogashira Reaction[J]. Acta Chimica Sinica, 2021, 79(11): 1385-1393.
| Entrya | Catalyst | CuI | Base | Yieldb/% |
|---|---|---|---|---|
| 1 | PdCl2(PPh3)2 | — | (iPr)2NH | Tracec |
| 2 | PdCl2(PPh3)2 | 10% | (iPr)2NH | 76 |
| 3 | PdCl2(PPh3)2 | 2.5% | (iPr)2NH | 84 |
| 4 | Pd-DPPF | 2.5% | (iPr)2NH | 76 |
| 5 | Pd(PPh3)4 | 2.5% | (iPr)2NH | Tracec |
| 6 | PdCl2(PPh3)2 | 2.5% | Et3N | Tracec |
| 7 | PdCl2(PPh3)2 | 2.5% | Pyridine | Tracec |
| 8 | PdCl2(PPh3)2 | 2.5% | K2CO3 | tracec |
| 9d | PdCl2(PPh3)2 | 2.5% | (iPr)2NH | 74 |
| 10e | PdCl2(PPh3)2 | 2.5% | (iPr)2NH | 65 |
| Entrya | Catalyst | CuI | Base | Yieldb/% |
|---|---|---|---|---|
| 1 | PdCl2(PPh3)2 | — | (iPr)2NH | Tracec |
| 2 | PdCl2(PPh3)2 | 10% | (iPr)2NH | 76 |
| 3 | PdCl2(PPh3)2 | 2.5% | (iPr)2NH | 84 |
| 4 | Pd-DPPF | 2.5% | (iPr)2NH | 76 |
| 5 | Pd(PPh3)4 | 2.5% | (iPr)2NH | Tracec |
| 6 | PdCl2(PPh3)2 | 2.5% | Et3N | Tracec |
| 7 | PdCl2(PPh3)2 | 2.5% | Pyridine | Tracec |
| 8 | PdCl2(PPh3)2 | 2.5% | K2CO3 | tracec |
| 9d | PdCl2(PPh3)2 | 2.5% | (iPr)2NH | 74 |
| 10e | PdCl2(PPh3)2 | 2.5% | (iPr)2NH | 65 |
| Entrya | R | Time/h | Yieldb/% | Complex |
|---|---|---|---|---|
| 1 | Ph | 10 | 84 | 2a |
| 2 | p-PhF | 10 | 72 | 2b |
| 3 | p-PhBr | 12 | 78 | 2c |
| 4 | p-PhEt | 11 | 70 | 2d |
| 5 | p-PhOMe | 12 | 82 | 2e |
| 6c | n-C3H7 | 12 | 82 | 2f |
| 7c | n-C6H13 | 7 | 76 | 2g |
| 8c | TMS | 11 | 80 | 2h |
| 9 | CH2OH | 72 | traced | 2i |
| 10 | CH2NMe2 | 72 | traced | 2j |
| Entrya | R | Time/h | Yieldb/% | Complex |
|---|---|---|---|---|
| 1 | Ph | 10 | 84 | 2a |
| 2 | p-PhF | 10 | 72 | 2b |
| 3 | p-PhBr | 12 | 78 | 2c |
| 4 | p-PhEt | 11 | 70 | 2d |
| 5 | p-PhOMe | 12 | 82 | 2e |
| 6c | n-C3H7 | 12 | 82 | 2f |
| 7c | n-C6H13 | 7 | 76 | 2g |
| 8c | TMS | 11 | 80 | 2h |
| 9 | CH2OH | 72 | traced | 2i |
| 10 | CH2NMe2 | 72 | traced | 2j |
| Entrya | R | Time/h | Yieldb/% | Complex |
|---|---|---|---|---|
| 1 | Ph | 7 | >99 | 3a |
| 2 | p-PhF | 8 | 88 | 3b |
| 3 | p-PhBr | 23 | 84 | 3c |
| 4 | p-PhEt | 24 | 86 | 3d |
| 5 | p-PhOMe | 7 | 91 | 3e |
| 6c | n-C3H7 | 30 | 91 | 3f |
| 7 c | n-C14H29 | 48 | 92 | 3g |
| 8c | TMS | 24 | 77 | 3h |
| 9 | CH2OH | 72 | 64d | 3i |
| 10 | CH2NMe2 | 72 | 74 | 3j |
| Entrya | R | Time/h | Yieldb/% | Complex |
|---|---|---|---|---|
| 1 | Ph | 7 | >99 | 3a |
| 2 | p-PhF | 8 | 88 | 3b |
| 3 | p-PhBr | 23 | 84 | 3c |
| 4 | p-PhEt | 24 | 86 | 3d |
| 5 | p-PhOMe | 7 | 91 | 3e |
| 6c | n-C3H7 | 30 | 91 | 3f |
| 7 c | n-C14H29 | 48 | 92 | 3g |
| 8c | TMS | 24 | 77 | 3h |
| 9 | CH2OH | 72 | 64d | 3i |
| 10 | CH2NMe2 | 72 | 74 | 3j |
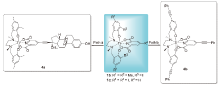

| Entrya | HeLa S3 | Hep G2 | Complex | Entrya | HeLa S3 | Hep G2 | Complex |
|---|---|---|---|---|---|---|---|
| 1 | 0.9±0.2 | 4.1±0.9 | 2a | 12 | 59.1±6.2 | Non toxic | 3d |
| 2 | 1.1±0.2 | 3.2±0.4 | 2b | 13 | Non toxic | Non toxic | 3e |
| 3 | 3.8±0.8 | 10.7±1 | 2c | 14 | 10.6±2 | 26.1±3.3 | 3f |
| 4 | 3.2±0.4 | 3.6±0.5 | 2d | 15 | Non toxic | Non toxic | 3g |
| 5 | 7.1±1.3 | 16.8±2.4 | 2e | 16 | 5.9±0.4 | 30.2±5.7 | 3h |
| 6 | 2.1±0.3 | 7.1±0.7 | 2f | 17 | 0.8±0.2 | 1.46±0.7 | 3i |
| 7 | 11.8±2.6 | 27.7±6.3 | 2g | 18 | 0.5±0.1 | 0.7±0.2 | 3j |
| 8 | 0.6±0.1 | 0.8±0.1 | 2h | 19 | Non toxic | Non toxic | 4a |
| 9 | 1.9±2.6 | 58.6±16 | 3a | 20 | 11.8±1.1 | Non-toxic | 4b |
| 10 | 1.1±0.2 | 6.5±1.3 | 3b | 20 | 3.3±0.2 | 6.0±1.1 | Cisplatin |
| 11 | 1.6±0.3 | 17.7±7.6 | 3c | 21[ | 4.4±0.4 | 3.4±0.2 | [L2Ti(IV)Dipic] |
| Entrya | HeLa S3 | Hep G2 | Complex | Entrya | HeLa S3 | Hep G2 | Complex |
|---|---|---|---|---|---|---|---|
| 1 | 0.9±0.2 | 4.1±0.9 | 2a | 12 | 59.1±6.2 | Non toxic | 3d |
| 2 | 1.1±0.2 | 3.2±0.4 | 2b | 13 | Non toxic | Non toxic | 3e |
| 3 | 3.8±0.8 | 10.7±1 | 2c | 14 | 10.6±2 | 26.1±3.3 | 3f |
| 4 | 3.2±0.4 | 3.6±0.5 | 2d | 15 | Non toxic | Non toxic | 3g |
| 5 | 7.1±1.3 | 16.8±2.4 | 2e | 16 | 5.9±0.4 | 30.2±5.7 | 3h |
| 6 | 2.1±0.3 | 7.1±0.7 | 2f | 17 | 0.8±0.2 | 1.46±0.7 | 3i |
| 7 | 11.8±2.6 | 27.7±6.3 | 2g | 18 | 0.5±0.1 | 0.7±0.2 | 3j |
| 8 | 0.6±0.1 | 0.8±0.1 | 2h | 19 | Non toxic | Non toxic | 4a |
| 9 | 1.9±2.6 | 58.6±16 | 3a | 20 | 11.8±1.1 | Non-toxic | 4b |
| 10 | 1.1±0.2 | 6.5±1.3 | 3b | 20 | 3.3±0.2 | 6.0±1.1 | Cisplatin |
| 11 | 1.6±0.3 | 17.7±7.6 | 3c | 21[ | 4.4±0.4 | 3.4±0.2 | [L2Ti(IV)Dipic] |


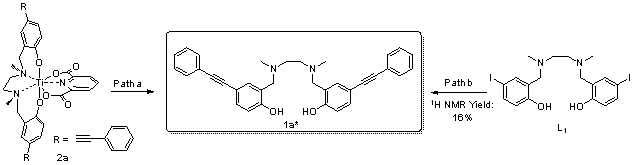
| [1] |
(a) Qi H.-T.; Song G.-L.; Quan Z.-J.; Wang X.-C. Chin. J. Org. Chem. 2017, 37, 1855. (in Chinese)
doi: 10.6023/cjoc201701013 |
|
( 戚海棠, 宋光琳, 权正军, 王喜存, 有机化学, 2017, 37, 1855.)
doi: 10.6023/cjoc201701013 |
|
|
(b) Dong X.-Y.; Zhang Y.-F.; Ma C.-L.; Gu Q.-S.; Wang F.-L.; Li Z.-L.; Jiang S.-P.; Liu X.-Y. Nat. Chem. 2019, 11, 1158.
doi: 10.1038/s41557-019-0346-2 |
|
|
(c) Chinchilla R.; Najera C. Chem. Rev. 2007, 38, 5506.
|
|
| [2] |
Wu S.; Zhu Z.; Liu C.; Su Y.; Wang F.; Bai W.; Sucn H.; Liang W.; Li A. J. Colloid Interface Sci. 2021, 586, 152.
doi: 10.1016/j.jcis.2020.10.080 |
| [3] |
Kim J. G.; Cha M. C.; Lee J.; Choi T.; Chang J. Y. ACS Appl. Mater. Inter. 2017, 9, 38081.
doi: 10.1021/acsami.7b14807 |
| [4] |
Corr M. J.; ShaV.; Pubill-Ulldemolins C.; Bown R. T.; Poirot P.; Smith D. R. M.; Cartmell C.; Abou Fayad A.; Goss R. J. M.; Chem. Sci. 2017, 8, 2039.
doi: 10.1039/c6sc04423a pmid: 28451322 |
| [5] |
(a) Ferrazzano L.; Martelli G.; Fantoni T.; Daka A.; Corbisiero D.; Viola A.; Ricci A.; Cabri W.; Tolomelli A. Org. Lett. 2020, 22, 3969.
doi: 10.1021/acs.orglett.0c01269 pmid: 32342693 |
|
(b) Zhang M.; Su W.-P.; Chin. J. Org. Chem. 2019, 39, 3596. (in Chinese)
doi: 10.6023/cjoc201900007 pmid: 32342693 |
|
|
( 张敏, 苏伟平, 有机化学, 2019, 39, 3596.)
doi: 10.6023/cjoc201900007 pmid: 32342693 |
|
| [6] |
(a) Bai Y.-L.; Li X.-W.; Xiao X.-D.; Liu J.-Q.; Yang J.-J.; Wang J.-W. Chin. J. Org. Chem. 2017, 37, 1258. (in Chinese)
doi: 10.6023/cjoc201610039 |
|
( 白亚丽, 李晓维, 肖雪冬, 刘佳琦, 杨俊娟, 王君文, 有机化学, 2017, 37, 1258.)
doi: 10.6023/cjoc201610039 |
|
|
(b) Wu X. F.; Neumann H.; Beller M. ChemSusChem 2013, 6, 229.
doi: 10.1002/cssc.v6.2 |
|
| [7] |
(a) Mondal A.; Chen H.; Flämig L.; Wedi P.; van Gemmeren M. J. Am. Chem. Soc. 2019, 141, 18662.
doi: 10.1021/jacs.9b10868 pmid: 31715100 |
|
(b) Balasingham R. G.; Williams C. F.; Mottram H. J.; Coogan M. P.; Pope S. J. A. Organometallics 2012, 31, 5835.
doi: 10.1021/om300475y pmid: 31715100 |
|
| [8] |
(a) Su Y. C.; Lo Y. L.; Hwang C. C.; Wang L. F.; Wu M. H.; Wang E. C.; Wang Y. M.; Wang T. P. Org. Biomol. Chem. 2014, 12, 6624.
doi: 10.1039/C4OB01132E pmid: 23356907 |
|
(b) Ranyuk E.; Cauchon N.; Klarskov K.; Guérin B.; van Lier J. E. J. Med. Chem. 2013, 56, 1520.
doi: 10.1021/jm301311c pmid: 23356907 |
|
| [9] |
Segura J. L.; Royuela S.; Mar Ramos M. Chem. Soc. Rev. 2019, 48, 3903.
doi: 10.1039/c8cs00978c pmid: 31187790 |
| [10] |
Liang L.; Niu H. Y.; Li R. L.; Wang Y. F.; Yan J. K.; Li C. G.; Guo H. M. Org. Lett. 2020, 22, 6842.
doi: 10.1021/acs.orglett.0c02364 pmid: 32810404 |
| [11] |
Cundari T. R.; Gordon M. S. J. Am. Chem. Soc. 1992, 114, 539.
doi: 10.1021/ja00028a022 |
| [12] |
Köpf H.; Köpf-Maier P. Angew. Chem. 1979, 91, 509.
doi: 10.1002/(ISSN)1521-3757 |
| [13] |
Schilling T.; Keppler K. B.; Heim M. E.; Niebch G.; Dietzfelbinger H.; Rastetter J.; Hanauske A. R. Invest. New Drugs 1995, 13, 327.
doi: 10.1007/BF00873139 |
| [14] |
Tshuva E. Y.; Miller M. Met. Ions Life Sci. 2018, 18. 219.
|
| [15] |
(a) Glasner H.; Tshuva E. Y. J. Am. Chem. Soc. 2011, 133, 16812.
doi: 10.1021/ja208219f pmid: 24588655 |
|
(b) Glasner H.; Tshuva E. Y. Inorg. Chem. 2014, 53, 3170.
doi: 10.1021/ic500001j pmid: 24588655 |
|
| [16] |
Manna C. M.; Braitbard O.; Weiss E.; Hochman J.; Tshuva E. Y. ChemMedChem 2012, 7, 703.
doi: 10.1002/cmdc.v7.4 |
| [17] |
(a) Pesch T.; Schuhwerk H.; Wyrsch P.; Immel T.; Dirks W.; Bürkle A.; Huhn T.; Beneke S. BMC Cancer 2016, 16, 469.
doi: 10.1186/s12885-016-2538-0 pmid: 32585595 |
|
(b) Miller M.; Braitbard O.; Hochman J.; Tshuva E. Y. J. Inorg. Biochem. 2016, 163, 250.
doi: S0162-0134(16)30089-7 pmid: 32585595 |
|
|
(c) Miller M.; Mellul A.; Braun M.; Sherill-Rofe D.; Cohen E.; Shpilt Z.; Unterman I.; Braitbard O.; Hochman J.; Tshuva E. Y.; Tabach Y. iScience 2020, 23, 101262.
doi: S2589-0042(20)30448-X pmid: 32585595 |
|
| [18] |
Meker S.; Margulis-Goshen K.; Weiss E.; Magdassi S. Angew. Chem., Int. Ed. 2012, 51, 10515.
doi: 10.1002/anie.201205973 |
| [19] |
(a) Shavit M.; Peri D.; Manna C. M.; Alexander J. S.; Tshuva E. Y. J. Am. Chem. Soc. 2007, 129, 12098.
doi: 10.1021/ja0753086 pmid: 22112842 |
|
(b) Immel T. A.; Grützke M.; Batroff E.; Groth U.; Huhn T. J. Inorg. Biochem. 2012, 106, 68.
doi: 10.1016/j.jinorgbio.2011.08.029 pmid: 22112842 |
|
| [20] |
Immel T. A.; Grützke M.; Spate A.-K.; Groth U.; Öhlschlager P.; Huhn T. Chem. Commun. 2012, 48, 5790.
doi: 10.1039/c2cc31624b |
| [21] |
Severin G. W.; Nielsen C. H.; Jensen A. I.; Fonslet J.; Kjær A.; Zhuravlev F. J. Med. Chem. 2015, 58, 7591.
doi: 10.1021/acs.jmedchem.5b01167 |
| [22] |
Søborg Pedersen K.; Baun C.; Michaelsen Nielsen K.; Thisgaard H.; Ingemann Jensen A.; Zhuravlev F. Molecules 2020, 25, 1104.
doi: 10.3390/molecules25051104 |
| [23] |
(a) Immel T. A.; Groth U.; Huhn T. Chem. Eur. J. 2010, 16, 2775.
doi: 10.1002/chem.200902312 |
|
(b) Djukic B.; Poddutoori P. K.; Dube P. A.; Seda T.; Jenkins H. A.; Lemaire M. T. Inorg. Chem. 2009, 48, 6109.
doi: 10.1021/ic9004938 |
|
|
(c) Storm O.; Lüning U. Eur. J. Org. Chem. 2002, 2002, 3680.
doi: 10.1002/1099-0690(200211)2002:21【-逻*辑*与-】#x00026;lt;3680::AID-EJOC3680【-逻*辑*与-】#x00026;gt;3.0.CO;2-4 |
|
| [24] |
Hannon M. J.; Green P. S.; Fisher D. M.; Derrick P. J.; Beck J. L.; Watt S. J.; Ralph S. F.; Sheil M. M.; Barker P. R.; Alcock N. W.; Price R. J.; Sanders K. J.; Pither R.; Davis J.; Rodger A. Chem. Eur. J. 2006, 12, 8000.
doi: 10.1002/(ISSN)1521-3765 |
| [25] |
Grützke M.; Zhao T.; Immel T. A.; Huhn T. Inorg. Chem. 2015, 54, 6697.
doi: 10.1021/acs.inorgchem.5b00690 pmid: 26151574 |
| [26] |
Zhao T.; Grützke M.; Götz K. H.; Druzhenko T.; Huhn T. Dalton Trans. 2015, 44, 16475.
doi: 10.1039/C5DT01618E |
| [27] |
Immel T. A.; Grützke M.; Späte A.-K.; Groth U.; Öhlschläger P.; Huhn T. Chem. Commun. 2012, 48, 5790.
doi: 10.1039/c2cc31624b |
| [28] |
Fields R. D.; Lancaster M. V. Am. Biotechnol. Lab 1993, 11, 48.
pmid: 7763491 |
| [29] |
Systat Software, Inc., 2006, http://www.systat.com.
|
| [1] | Xuelu Ma, Meng Li, Ming Lei. Trinuclear Transition Metal Complexes in Catalytic Reactions [J]. Acta Chimica Sinica, 2023, 81(1): 84-99. |
| [2] | Liu Qiyan, Cai Daihong, Qi Yongyu, Le Xueyi. DNA Interaction and Antitumor Activity of A Copper(II) Complex Containing Sparfloxacin and Triazine Derivatives [J]. Acta Chimica Sinica, 2020, 78(3): 263-270. |
| [3] | Chen Fangyuan, Qu Ning, Wu Qunyan, Zhang Hongxing, Shi Weiqun, Pan Qingjiang. Structures and Uranium-Uranium Multiple Bond of Binuclear Divalent Uranium Complex of Pyrrolic Schiff-base Macrocycle: a Relativistic DFT Probe [J]. Acta Chim. Sinica, 2017, 75(5): 457-463. |
| [4] | Zhou Qianxiong, Wang Xuesong. Advances in Ru(II)-based Photoactivated Chemotherapy Agents [J]. Acta Chim. Sinica, 2017, 75(1): 49-59. |
| [5] | Cui Bin-Bin, Tang Jian-Hong, Zhong Yu-Wu. Resistive Memory Materials Based on Transition-Metal Complexes [J]. Acta Chim. Sinica, 2016, 74(9): 726-733. |
| [6] | Zhang Yiwe, Ma Xuelu, Zhang Xin, Lei Ming. Theoretical Study on N-N Activation by Thiolate-bridged Dinuclear Dinitrogen Transition-metal Complexes [J]. Acta Chim. Sinica, 2016, 74(4): 340-350. |
| [7] | Liu Ying, Chen Xiaoman, Zhang Langqi, Sun Dongdong, Zhou Yanhui, Chen Lanmei, Liu Jie. Stabilization of Telomere DNA, and Mechanism of Apoptosis of Tumor Cells Induced by Ruthenium Complexes [J]. Acta Chimica Sinica, 2014, 72(4): 473-480. |
| [8] | Qiu Yixiang, Wang Shuguang. Theoretical Investigations on Tris(pentamethylcyclopentadienyl) Rare Earth Metal Complexes (C5Me5)3Ln (Ln=Sc, Y, La) [J]. Acta Chimica Sinica, 2012, 70(18): 1930-1938. |
| [9] | Zheng Ke, Lin Lili, Feng Xiaoming. Chiral N,N '-Dioxide-Ni(II) Complex Catalyzed Asymmetric Carbonyl-Ene Reaction of Ethyl Trifluoropyruvate [J]. Acta Chimica Sinica, 2012, 70(17): 1785-1790. |
| [10] | Liu Haibin, Xu Huijuan, L? Ping, Pan Ningning, Li Shuangqi. Synthesis and in vitro Antitumor Activities of 6,7-Dimethoxy-4-piperazinquinazoline Thiosemicarbazone Derivatives [J]. Acta Chimica Sinica, 2012, 0(05): 674-678 . |
| [11] | SHI Yan-Ping, CHEN Bao-Quan, MA Jing, LIU Yu-Ming, LI Cai-Wen. Synthesis and Antitumor Activity of 2-(2-Substituted 1,3,4-thiadiazol-5-yl)benzisoselenazol-3(2H)-one Derivatives [J]. Acta Chimica Sinica, 2011, 69(21): 2561-2566. |
| [12] | ZHOU Shuang-Sheng, XUE Xuan, JIANG Bo, LU Chuan-Hua, TIAN Yu-Peng, JIANG Ming-Hua. Synthesis and Antitumor Activities of Platinum(II) Complexes of Curcumin Analog [J]. Acta Chimica Sinica, 2011, 69(19): 2335-2340. |
| [13] | ZHAO Fang, LIANG Hui, CHENG Hui, WANG Jun. Synthesis, Characterization and Antioxidative Activity of Metal Complexes with Rhein [J]. Acta Chimica Sinica, 2011, 69(08): 925-930. |
| [14] | MA Yong-Jun, YANG Mei-Xia, WANG Wei-Feng, ZHOU Min, LIU Jing, LI Ting-Ting, CHEN Hui. Electrocatalytic Oxidation Behavior of Formic Acid at Platinum Electrode Modified with Nd-Fe-WO 42-Cyanide-bridged Mixed Complexes [J]. Acta Chimica Sinica, 2011, 69(03): 262-268. |
| [15] | LIU Yan, CHOU Yong-Qing, SUN Shi-Ling, ZHAO Hai-Bo, SU Zhong-Min. Density Functional Theory Study On Second-order Nonlinear Optical Properties of Tetrathiafulvalene-Fused Schiff Base Ligand and Complexes [J]. Acta Chimica Sinica, 2010, 68(14): 1443-1448. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||