Acta Chimica Sinica ›› 2023, Vol. 81 ›› Issue (11): 1522-1540.DOI: 10.6023/A23070336 Previous Articles Next Articles
Special Issue: 庆祝《化学学报》创刊90周年合辑
Perspective
投稿日期:2023-07-13
发布日期:2023-09-11
作者简介: |
韩叶强, 2016年毕业于浙江工业大学获学士学位, 之后在浙江大学史炳锋教授的指导下攻读博士学位并在2021年获得理学博士学位, 2021年7月加入黄飞鹤教授课题组从事博士后研究. 主要研究方向为不对称碳氢键活化和新型大环体系的高效构筑. |
 |
史炳锋, 博士, 教授, 独立课题组组长. 2001年本科毕业于南开大学化学系, 2006年博士毕业于中科院上海有机所. 2006年到2007年在University of California, San Diego从事博士后研究, 2007年到2010年加入The Scripps Research Institute从事博士后研究. 2010年4月加入浙江大学化学系, 任独立课题组组长, 博士生导师, 主要研究领域为过渡金属催化的惰性键活化及其在天然产物全合成中的应用. |
基金资助:
Yeqiang Hana, Bingfeng Shia,b( )
)
Received:2023-07-13
Published:2023-09-11
Contact:
*E-mail: About author:Supported by:Share
Yeqiang Han, Bingfeng Shi. Palladium(II)-Catalyzed Enantioselective Functionalization of C(sp3)—H Bonds★[J]. Acta Chimica Sinica, 2023, 81(11): 1522-1540.
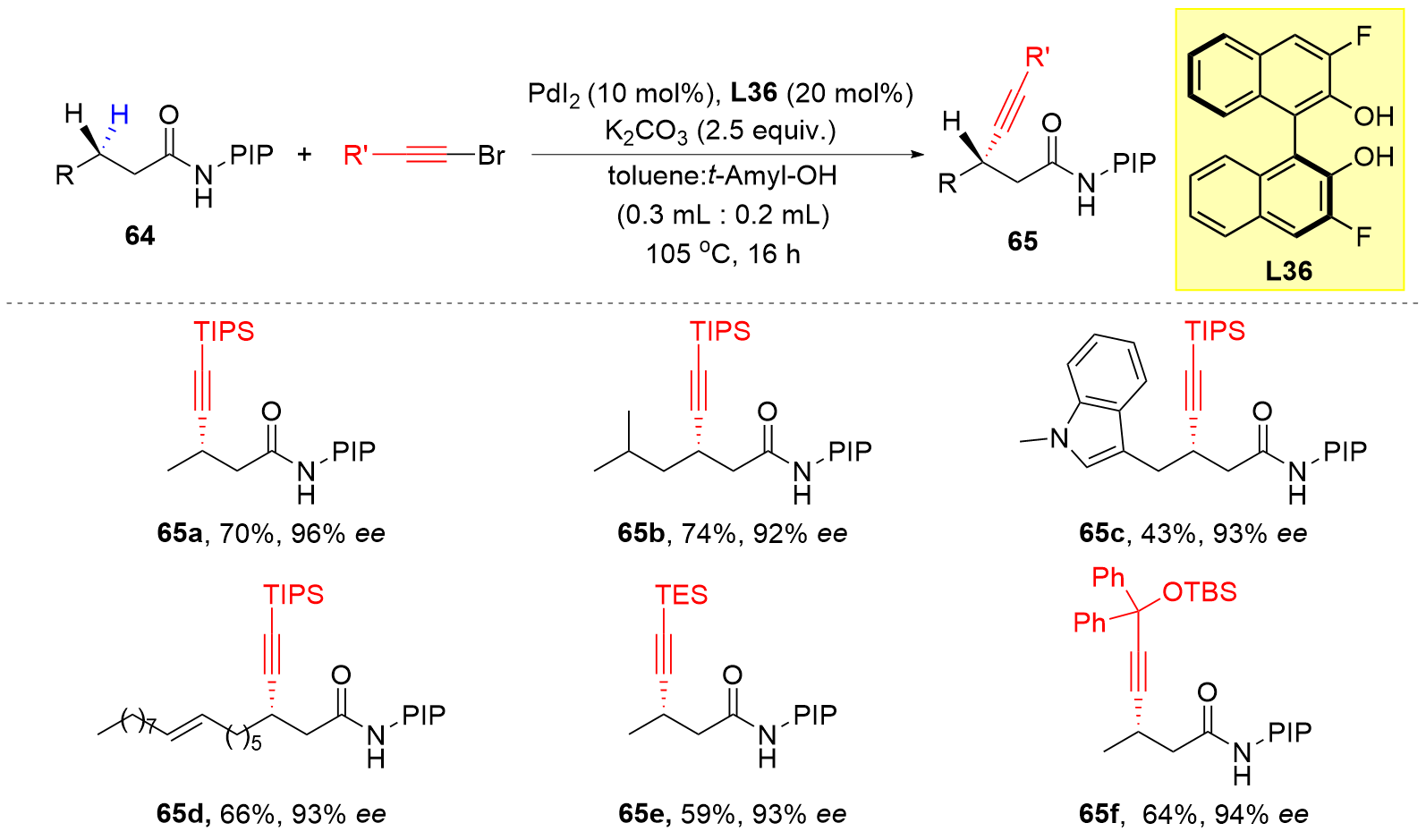
| [1] |
(a) Godula K.; Sames D. Science 2006, 312, 67.
pmid: 16601184 |
|
(b) Chen X.; Engle K. M.; Wang D.-H.; Yu J.-Q. Angew. Chem., Int. Ed. 2009, 48, 5094.
doi: 10.1002/anie.v48:28 pmid: 16601184 |
|
|
(b) Lyons T. W.; Sanford M. S. Chem. Rev. 2010, 110, 1147.
doi: 10.1021/cr900184e pmid: 16601184 |
|
|
(c) Baudoin O. Chem. Soc. Rev. 2011, 40, 4902.
doi: 10.1039/c1cs15058h pmid: 16601184 |
|
|
(d) Rago A. J.; Dong G. Green Synth. Catal. 2021, 2, 216.
pmid: 16601184 |
|
|
(e) Tong H.-R.; Li B.; Li G.; He G.; Chen G. CCS Chem. 2021, 3, 1797.
doi: 10.31635/ccschem.020.202000426 pmid: 16601184 |
|
|
(f) Shi L.; Li T.; Mei G.-J. Green Synth. Catal. 2022, 3, 227.
pmid: 16601184 |
|
|
(g) Tang L.; Hu Q.; Yang K.; Elsaid M.; Liu C.; Ge H. Green Synth. Catal. 2022, 3, 203.
pmid: 16601184 |
|
|
(h) Newton C. G.; Wang S.-G.; Oliveira C. C.; Cramer N. Chem. Rev. 2017, 117, 8908.
doi: 10.1021/acs.chemrev.6b00692 pmid: 16601184 |
|
| [2] |
(a) Pan F.; Shi Z. Acta Chim. Sinica 2012, 70, 1679. (in Chinese)
doi: 10.6023/A12040135 |
|
( 潘菲, 施章杰, 化学学报, 2012, 70, 1679.)
doi: 10.6023/A12040135 |
|
|
(b) Huang J.; Gu Q.; You S.-L. Chin. J. Org. Chem. 2018, 38, 51. (in Chinese)
doi: 10.6023/cjoc201708030 |
|
|
( 黄家翩, 顾庆, 游书力, 有机化学, 2018, 38, 51.)
doi: 10.6023/cjoc201708030 |
|
|
(c) Cai Y.; Jiao L.; Qiu X.; Du Z. Chem. Res. Chinese U. 2020, 36, 843.
doi: 10.1007/s40242-019-9013-9 |
|
|
(d) Liao G.; Wu Y.-J.; Shi B.-F. Acta Chim. Sinica, 2020, 78, 289. (in Chinese)
doi: 10.6023/A20020027 |
|
|
( 廖港, 吴勇杰, 史炳锋, 化学学报, 2020, 78, 289.)
doi: 10.6023/A20020027 |
|
|
(e) Cheng X.; Lei A.; Mei T.-S.; Xu H.-C.; Xu K.; Zeng C. CCS Chem. 2022, 4, 1120.
doi: 10.31635/ccschem.021.202101451 |
|
|
(f) Xiang X.; He Z.; Dong X. Chin. J. Org. Chem. 2023, 43, 791. (in Chinese)
doi: 10.6023/cjoc202211043 |
|
|
向勋, 何照林, 董秀琴, 有机化学, 2023, 43, 791).
|
|
|
(g) Chen J.-H.; Shi B.-F. Chin. J. Org. Chem. 2022, 42, 2999. (in Chinese)
doi: 10.6023/cjoc202200047 |
|
|
( 陈嘉豪, 史炳锋, 有机化学, 2022, 42, 2999.)
doi: 10.6023/cjoc202200047 |
|
|
(h) Wang F.; Li X. Chin. J. Org. Chem. 2022, 42, 4350. (in Chinese)
doi: 10.6023/cjoc202200071 |
|
|
( 王芬, 李兴伟, 有机化学, 2022, 42, 4350.)
doi: 10.6023/cjoc202200071 |
|
|
(i) Chen J.-H.; Shi B.-F. Chin. J. Org. Chem. 2023, 43, 2252. (in Chinese)
doi: 10.6023/cjoc202300031 |
|
|
( 陈嘉豪, 史炳锋, 有机化学, 2023, 43, 2252.)
doi: 10.6023/cjoc202300031 |
|
| [3] |
(a) Giri R.; Shi B.-F.; Engle K. M.; Maugel N.; Yu J.-Q. Chem. Soc. Rev. 2009, 38, 3242.
doi: 10.1039/b816707a |
|
(b) You S.-L. Asymmetric Functionalization of C-H Bonds, Royal Society of Chemistry, Cambridge, 2015.
|
|
|
(c) Newton C. G.; Wang S.-G.; Oliveira C. C.; Cramer N. Chem. Rev. 2017, 117, 8908.
doi: 10.1021/acs.chemrev.6b00692 |
|
|
(d) Wang Q.; Gu Q.; You S.-L. Acta Chim. Sinica 2019, 77, 690. (in Chinese)
doi: 10.6023/A19060222 |
|
|
( 王强, 顾庆, 游书力, 化学学报, 2019, 77, 690.)
doi: 10.6023/A19060222 |
|
|
(e) Liao G.; Zhang T.; Liu Z.-K.; Shi B.-F. Angew. Chem., Int. Ed. 2020, 59, 19773.
doi: 10.1002/anie.v59.45 |
|
|
(f) Achar T.; Maiti S.; Jana S.; Maiti D. ACS Catal. 2020, 10, 13748.
doi: 10.1021/acscatal.0c03743 |
|
|
(g) Du Y.; Huang Y.; Gao Y.; Shen H.; Su W.; Hong M. Sci. Sin.-Chim. 2021, 51, 123. (in Chinese)
doi: 10.1360/SSC-2020-0192 |
|
|
( 杜宇, 黄永亮, 高越, 沈慧, 苏伟平, 洪茂椿, 中国科学: 化学, 2021, 51, 123.)
|
|
|
(h) Yu X.; Zhang Z.-Z.; Niu J.-L.; Shi B.-F. Org. Chem. Front. 2022, 9, 1458.
doi: 10.1039/D1QO01884A |
|
| [4] |
(a) Hayashi H.; Uchida T. Eur. J. Org. Chem., 2020, 2020, 909.
doi: 10.1002/ejoc.v2020.8 |
|
(b) Xia Y.; Qiu D.; Wang J. Chem. Rev. 2017, 117, 13810.
doi: 10.1021/acs.chemrev.7b00382 |
|
|
(c) Davies H. M. L.; Manning J. R. Nature 2008, 451, 417.
doi: 10.1038/nature06485 |
|
| [5] |
(a) Wang F.; Chen P.; Liu G. Acc. Chem. Res. 2018, 51, 2036.
doi: 10.1021/acs.accounts.8b00265 pmid: 33473126 |
|
(b) Costa M. Chem. Rec. 2021, 21, 4000.
doi: 10.1002/tcr.v21.12 pmid: 33473126 |
|
|
(c) Zhang Z.; Chen P.; Liu G. Chem. Soc. Rev. 2022, 51, 1640.
doi: 10.1039/D1CS00727K pmid: 33473126 |
|
|
(d) Zhang C.; Li Z.-L.; Gu Q.-S.; Liu X.-Y. Nat. Commun. 2021, 12, 475.
doi: 10.1038/s41467-020-20770-4 pmid: 33473126 |
|
| [6] |
(a) Saint-Denis T. G.; Zhu R.-Y.; Chen G.; Wu Q.-F.; Yu J.-Q. Science 2018, 359, 759.
|
|
(b) Zhan B.-B.; Jin L.; Shi B.-F. Trends in Chem. 2022, 4, 220.
doi: 10.1016/j.trechm.2021.12.005 |
|
| [7] |
(a) Vyhivskyi O.; Kudashev A.; Miyakoshi T.; Baudoin O. Chem. Eur. J. 2021, 27, 1231;
doi: 10.1002/chem.v27.4 |
|
(b) Wang P.-S.; Gong L.-Z. Acc. Chem. Res. 2020, 53, 2841.
doi: 10.1021/acs.accounts.0c00477 |
|
|
(c) Wang P.-S.; Gong L.-Z. Chin. J. Chem. 2023, 41, 1841.
doi: 10.1002/cjoc.v41.15 |
|
|
(d) Wang P.-S.; Gong L.-Z. Synthesis 2022, 54, 4795.
doi: 10.1055/a-1662-7096 |
|
| [8] |
(a) Wang H.; Xu Y.; Zhang F.; Liu Y.; Feng X. Angew. Chem., Int. Ed. 2022, 61, e202115715.
doi: 10.1002/anie.v61.12 |
|
(b) Liu X.; Dong S.; Lin L.; Feng X. Chin. J. Chem. 2018, 36, 791.
doi: 10.1002/cjoc.v36.9 |
|
|
(c) Cao W.; Liu X.; Feng X. Chin. Sci. Bull. 2020, 65, 2941.
doi: 10.1360/TB-2020-0158 |
|
|
(d) Liu X.; Zheng H.; Xia Y.; Lin L.; Feng X. Acc. Chem. Res. 2017, 50, 2621.
doi: 10.1021/acs.accounts.7b00377 |
|
|
(e) Liu X.; Lin L.; Feng X. Org. Chem. Fron. 2014, 1, 298.
|
|
|
(f) Liu X.; Lin L.; Feng X. Acc. Chem. Res. 2011, 44, 574.
doi: 10.1021/ar200015s |
|
| [9] |
(a) Shi B.-F.; Maugel N.; Zhang Y.-H.; Yu J.-Q. Angew. Chem., Int. Ed. 2008, 47, 4882.
doi: 10.1002/anie.v47:26 |
|
(b) Wu Q.-F.; Shen P.-X.; He J.; Wang X.-B.; Zhang F.; Shao Q.; Zhu R.-Y.; Mapelli C.; Qiao J. X.; Poss M. A.; Yu J-Q. Science 2017, 355, 499.
doi: 10.1126/science.aal5175 |
|
| [10] |
Jiang H.-J.; Zhong X.-M.; Liu Z. Y.; Geng R. L.; Li Y. Y.; Wu Y.-D.; Zhang X.; Gong L.-Z. Angew. Chem., Int. Ed. 2020, 59, 12774.
doi: 10.1002/anie.v59.31 |
| [11] |
Yuan C.; Wang X.; Jiao L. Angew. Chem., Int. Ed. 2023, 62, e202300854.
doi: 10.1002/anie.v62.17 |
| [12] |
Shao Q.; Wu Q.-F.; He J.; Yu J.-Q. J. Am. Chem. Soc. 2018, 140, 5322.
doi: 10.1021/jacs.8b01094 |
| [13] |
He J.; Shao Q.; Wu Q.; Yu J.-Q. J. Am. Chem. Soc. 2017, 139, 3344.
doi: 10.1021/jacs.6b13389 |
| [14] |
Smalley A. P.; Cuthbertson J. D.; Gaunt M. J. J. Am. Chem. Soc. 2017, 139, 1412.
doi: 10.1021/jacs.6b12234 pmid: 28064488 |
| [15] |
Jiang H.-J.; Geng R.-L.; Wei J.-H; Gong L.-Z. Chin. J. Chem. 2021, 39, 3269.
doi: 10.1002/cjoc.v39.12 |
| [16] |
Uchikura T.; Kato S.; Makino Y.; Fujikawa M. J.; Yamanaka M.; Akiyama T. J. Am. Chem. Soc. 2023, 145, 15906.
doi: 10.1021/jacs.3c03552 |
| [17] |
Wasa M.; Engle K. M.; Lin D. W.; Yoo E. J.; Yu J.-Q. J. Am. Chem. Soc. 2011, 133, 19598.
doi: 10.1021/ja207607s |
| [18] |
Xiao K.-J.; Lin D. W.; Miura M.; Zhu R.-Y.; Gong W.; Wasa M.; Yu J.-Q. J. Am. Chem. Soc. 2014, 136, 8138.
doi: 10.1021/ja504196j |
| [19] |
Chan K. S. L.; Fu H.-Y.; Yu J.-Q. J. Am. Chem. Soc. 2015, 137, 2042.
doi: 10.1021/ja512529e |
| [20] |
Wu Q.-F.; Wang X.-B.; Shen P.-X.; Yu J.-Q. ACS Catal. 2018, 8, 2577.
doi: 10.1021/acscatal.8b00069 |
| [21] |
Xiao L.-J.; Hong K.; Luo F.; Hu L.; Ewing W. R.; Yeung K.-S.; Yu J.-Q. Angew. Chem., Int. Ed. 2020, 59, 9594.
doi: 10.1002/anie.v59.24 |
| [22] |
Zhuang Z.; Yu J.-Q. J. Am. Chem. Soc. 2020, 142, 12015.
doi: 10.1021/jacs.0c04801 pmid: 32605367 |
| [23] |
Shen P.-X.; Hu L.; Shao Q.; Hong K.; Yu J.-Q. J. Am. Chem. Soc. 2018, 140, 6545.
doi: 10.1021/jacs.8b03509 |
| [24] |
Hu L.; Shen P.-X.; Shao Q.; Hong K.; Qiao J. X.; Yu J.-Q. Angew. Chem., Int. Ed. 2019, 58, 2134.
doi: 10.1002/anie.v58.7 |
| [25] |
Jain P.; Verma P.; Xia G.; Yu J.-Q. Nat. Chem. 2017, 9, 140.
doi: 10.1038/nchem.2619 |
| [26] |
Jiang H.-J.; Zhong X.-M.; Yu J.; Zhang Y.; Zhang X.; Wu Y.-D.; Gong L.-Z. Angew. Chem., Int. Ed. 2019, 58, 1803.
doi: 10.1002/anie.v58.6 |
| [27] |
Zhang F.-L.; Hong K.; Li T.-J.; Park H.; Yu J.-Q. Science 2016, 351, 252.
doi: 10.1126/science.aad7893 |
| [28] |
Park H.; Verma P.; Hong K.; Yu J.-Q. Nat. Chem. 2018, 10, 755.
doi: 10.1038/s41557-018-0048-1 |
| [29] |
Yan S.-B.; Zhang S.; Duan W.-L. Org. Lett. 2015, 17, 2458.
doi: 10.1021/acs.orglett.5b00968 |
| [30] |
Tong H.-R.; Zheng S.; Li X.; Deng Z.; Wang H.; He G.; Peng Q.; Chen G. ACS Catal. 2018, 8, 11502.
doi: 10.1021/acscatal.8b03654 |
| [31] |
Wang H.; Tong H.-R.; He G.; Chen G. Angew. Chem., Int. Ed. 2016, 55, 15387.
doi: 10.1002/anie.v55.49 |
| [32] |
Chen G.; Gong W.; Zhuang Z.; Andrä M. S.; Chen Y.-Q.; Hong X.; Yang Y.-F.; Liu T.; Houk K. N.; Yu J.-Q. Science 2016, 353, 1023.
doi: 10.1126/science.aaf4434 |
| [33] |
Yang Y.-F.; Chen G.; Hong X.; Yu J.-Q.; Houk K. N. J. Am. Chem. Soc. 2017, 139, 8514.
doi: 10.1021/jacs.7b01801 |
| [34] |
Li H.-L.; Yang D.-F.; Jing H.-Q.; Antilla J. C.; Kuninobu Y. Org. Lett. 2022, 24, 1286.
doi: 10.1021/acs.orglett.1c04215 |
| [35] |
(a) Zhang Q.; Chen K.; Rao W.-H.; Zhang Y.; Chen F.-J.; Shi B.-F. Angew. Chem. Int. Ed. 2013, 52, 13588.
doi: 10.1002/anie.201306625 pmid: 24174011 |
|
(b) Chen F.-J.; Zhao S.; Hu F.; Chen K.; Zhang Q.; Zhang S.-Q.; Shi B.-F. Chem. Sci. 2013, 4, 4187.
doi: 10.1039/c3sc51993g pmid: 24174011 |
|
|
(c) Zhang Qi; Shi B.-F. Chin. J. Chem. 2019, 37, 647.
doi: 10.1002/cjoc.v37.7 pmid: 24174011 |
|
|
(d) Zhang Q.; Shi B.-F. Acc. Chem. Res. 2021, 54, 2750.
doi: 10.1021/acs.accounts.1c00168 pmid: 24174011 |
|
| [36] |
Yan S.-Y.; Han Y.-Q.; Yao Q.-J.; Nie X.-L.; Liu L.; Shi B.-F. Angew. Chem., Int. Ed. 2018, 57, 9093.
doi: 10.1002/anie.v57.29 |
| [37] |
Han Y.-Q.; Ding Y.; Zhou T.; Yan S.-Y.; Song H.; Shi B.-F. J. Am. Chem. Soc. 2019, 141, 4558.
doi: 10.1021/jacs.9b01124 |
| [38] |
Zhou T.; Jiang M. X.; Yang X.; Yue Q.; Han Y.-Q.; Ding Y.; Shi B.-F. Chin. J. Chem. 2020, 38, 242.
doi: 10.1002/cjoc.v38.3 |
| [39] |
Tong H.-R.; Zheng W.; Lv X.; He G.; Liu P.; Chen G. ACS Catal. 2019, 10, 114.
doi: 10.1021/acscatal.9b04768 |
| [40] |
Ding Y.; Han Y.-Q.; Wu L.-S.; Zhou T.; Yao Q.-J.; Feng Y.-L.; Li Y.; Kong K.-X.; Shi B.-F. Angew. Chem., Int. Ed. 2020, 59, 14060.
doi: 10.1002/anie.v59.33 |
| [41] |
Han Y.-Q.; Zhang Q.; Yang X.; Jiang M. X.; Ding Y.; Shi B.-F. Org. Lett. 2021, 23, 97.
doi: 10.1021/acs.orglett.0c03775 |
| [42] |
Jiang M. X.; Yang X.; Han Y.-Q.; Zhou T.; Xu X.-T.; Zhang K.; Shi B.-F. Org. Chem. Front. 2021, 8, 2903.
doi: 10.1039/D1QO00302J |
| [43] |
Yang X.; Jiang M.-X.; Zhou T.; Han Y.-Q.; Xu X.-T.; Zhang K.; Shi B.-F. Chem. Commun. 2021, 57, 5562.
doi: 10.1039/D1CC01690C |
| [44] |
Andrä M. S.; Schifferer L.; Pollok C. H.; Merten C.; Gooßen L. J.; Yu J.-Q. Chem. Eur. J. 2019, 25, 8503.
doi: 10.1002/chem.v25.36 |
| [45] |
Wasa M.; Chan K. S. L.; Zhang X.-G.; He J.; Miura M.; Yu J.-Q. J. Am. Chem. Soc. 2012, 134, 18570.
doi: 10.1021/ja309325e |
| [46] |
Nack W. A.; Wang B.; Wu X.; Jiao R.; He G.; Chen G. Org. Chem. Front. 2016, 3, 561.
doi: 10.1039/C5QO00421G |
| [47] |
Han Y.-Q.; Yang X.; Kong K.-X.; Deng Y. T.; Wu L.-S.; Ding Y.; Shi B.-F. Angew. Chem., Int. Ed. 2020, 59, 20455.
doi: 10.1002/anie.v59.46 |
| [48] |
Wu L.-S.; Ding Y.; Han Y.-Q.; Shi B.-F. Org. Lett. 2021, 23, 2048.
doi: 10.1021/acs.orglett.1c00204 pmid: 33683896 |
| [49] |
Zhang Q.; Shi B.-F. Chem. Sci. 2021, 12, 841.
doi: 10.1039/D0SC05944G |
| [1] | Dawei Zhang, Haiyang Zhao, Xiaotian Feng, Yucheng Gu, Xingang Zhang. Palladium-Catalyzed Cross-Coupling of Heteroaryl Bromides with gem-Difluoroallylborons [J]. Acta Chimica Sinica, 2024, 82(2): 105-109. |
| [2] | Xiaomao Tian, Yuequn Lin, Han Zhu, Chao Huang, Bixue Zhu. Enantiomers Identification of Penicillamine by Chiral Mono-Schiff Base Macrocycles [J]. Acta Chimica Sinica, 2023, 81(1): 20-28. |
| [3] | Yixiu Ge, Zaozao Qiu, Zuowei Xie. Pd-Catalyzed One-Pot Synthesis of Difunctionalized o-Carboranes via Construction of B—C and B—Heteroatom Bonds※ [J]. Acta Chimica Sinica, 2022, 80(4): 432-437. |
| [4] | Yunfang Xu, Yang Li, Zitong Fu, Shaoyan Lin, Jie Zhu, Lei Wu. Palladium-catalyzed Stereoselective Synthesis of (Z)-[3]Dendralenes [J]. Acta Chimica Sinica, 2022, 80(10): 1369-1375. |
| [5] | Pusu Yang, Chen-Xu Liu, Wen-Wen Zhang, Shu-Li You. Ir-Catalyzed Enantioselective Friedel-Crafts Type Allylic Substitution of Indolizines [J]. Acta Chimica Sinica, 2021, 79(6): 742-746. |
| [6] | Zhuoji Deng, Yifan Ouyang, Yunlin Ao, Qian Cai. Copper(I)-Catalyzed Asymmetric Desymmetric Intramolecular Alkenyl C—N Coupling Reaction [J]. Acta Chimica Sinica, 2021, 79(5): 649-652. |
| [7] | Bo Zhou, Renxiao Liang, Zhongyan Cao, Pinghai Zhou, Yixia Jia. Palladium-Catalyzed Heck Reaction of Endocyclic Conjugated C=C Bonds of Pyrroles [J]. Acta Chimica Sinica, 2021, 79(2): 176-179. |
| [8] | Liao Gang, Wu Yong-Jie, Shi Bing-Feng. Noncovalent Interaction in Transition Metal-Catalyzed Selective C-H Activation [J]. Acta Chimica Sinica, 2020, 78(4): 289-298. |
| [9] | Zhang Ronghua, Xu Bing, Zhang Zhanming, Zhang Junliang. Ming-Phos/Copper(I)-Catalyzed Asymmetric[3+2] Cycloaddition of Azomethine Ylides with Nitroalkenes [J]. Acta Chimica Sinica, 2020, 78(3): 245-249. |
| [10] | Xiao, Yingxia, Liu, Zhong-Quan. Radical-Promoted Cross Dehydrogenative Coupling of Ketones and Esters with Electron-Rich Heteroarenes [J]. Acta Chimica Sinica, 2019, 77(9): 874-878. |
| [11] | Yang, Qi-Liang, Wang, Xiang-Yang, Weng, Xin-Jun, Yang, Xiang, Xu, Xue-Tao, Tong, Xiaofeng, Fang, Ping, Wu, Xin-Yan, Mei, Tian-Sheng. Palladium-Catalyzed ortho-Selective C—H Chlorination of Arenes Using Anodic Oxidation [J]. Acta Chimica Sinica, 2019, 77(9): 866-873. |
| [12] | Li Zhong-Yuan, Jing Kun, Li Qi-Li, Wang Guan-Wu. Palladium-Catalyzed Decarboxylative Coupling of Potassium Oxalate Monoester with 2-Aryloxypyridines [J]. Acta Chim. Sinica, 2019, 77(8): 729-734. |
| [13] | Khan Ijaz, Li Hongfang, Wu Xue, Zhang Yong Jian. Asymmetric Decarboxylative Cycloaddition of Vinylethylene Carbonates with Aldehydes by Cooperative Catalysis of Palladium Complex and Chiral Squaramide [J]. Acta Chim. Sinica, 2018, 76(11): 874-877. |
| [14] | Zhou Xiao-Le, Su Yong-Liang, Wang Pu-Sheng, Gong Liu-Zhu. Asymmetric Allylic C-H Alkylation of 1,4-Dienes with Aldehydes [J]. Acta Chim. Sinica, 2018, 76(11): 857-861. |
| [15] | Yu Yue-Na, Xu Ming-Hua. Chiral Phosphorus-Olefin Ligands for Asymmetric Catalysis [J]. Acta Chim. Sinica, 2017, 75(7): 655-670. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||