Acta Chimica Sinica ›› 2024, Vol. 82 ›› Issue (9): 925-931.DOI: 10.6023/A24060183 Previous Articles Next Articles
Article
投稿日期:2024-06-03
发布日期:2024-08-08
作者简介:基金资助:
Suhao Wang†, Mingxia Hu†, Hui Chen, Yanying Zhao*( )
)
Received:2024-06-03
Published:2024-08-08
Contact:
*E-mail: About author:Supported by:Share
Suhao Wang, Mingxia Hu, Hui Chen, Yanying Zhao. Synthesis, Structure and Photophysical Fluorescence Mechanism of Quinacridone Molecules[J]. Acta Chimica Sinica, 2024, 82(9): 925-931.

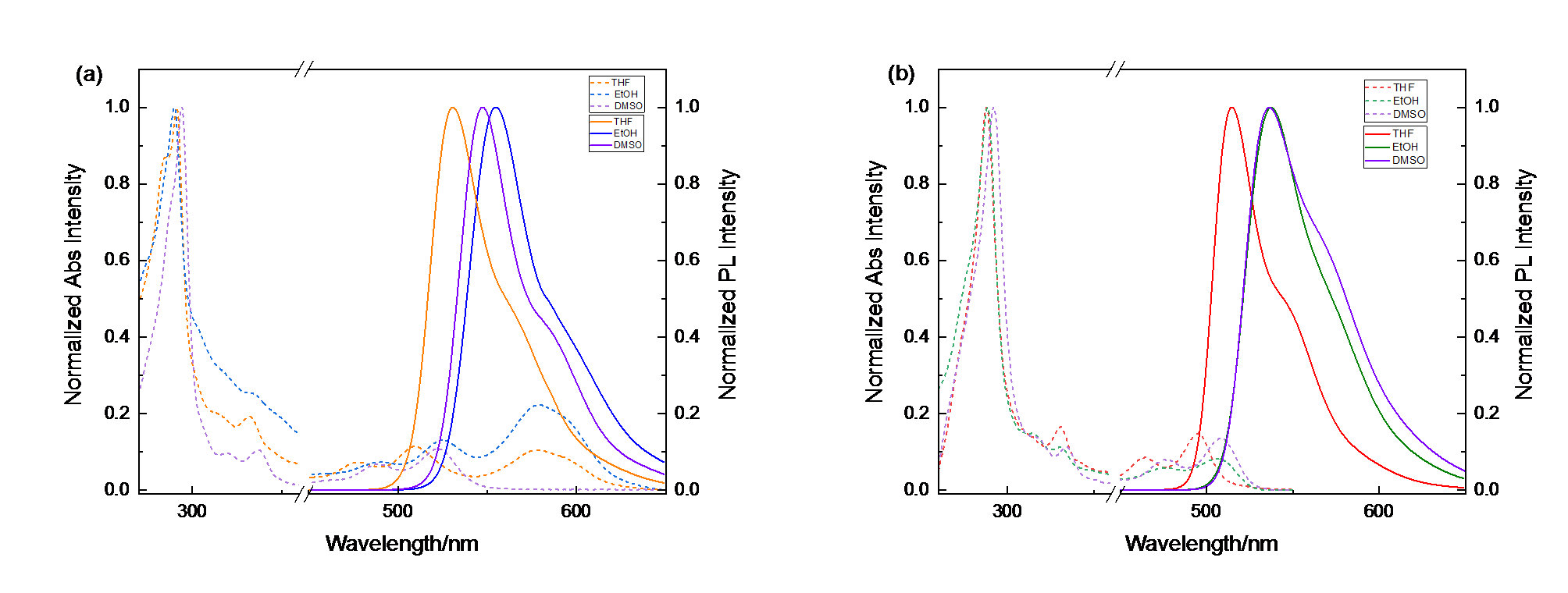
| Solvent | $\lambda _{abs}^{\max }$/nm | $\lambda _{\text{em}}^{\max }$/nm | |||
|---|---|---|---|---|---|
| QA | QA-CF3 | QA | QA-CF3 | ||
| THF | 291.2/332.0/478.0/509.6/577.8 | 288.0/330.8/462.2/494.8 | 530.4 | 514.2 | |
| EtOH | 289.9/330.3/490.0/525.5/579.1 | 288.4/330.6/477.8/505.2 | 554.4 | 537.0 | |
| DMSO | 294.2/336.8/488.0/522.6 | 292.0/334.2/477.2/509.0 | 547.0 | 536.0 | |
| Solvent | $\lambda _{abs}^{\max }$/nm | $\lambda _{\text{em}}^{\max }$/nm | |||
|---|---|---|---|---|---|
| QA | QA-CF3 | QA | QA-CF3 | ||
| THF | 291.2/332.0/478.0/509.6/577.8 | 288.0/330.8/462.2/494.8 | 530.4 | 514.2 | |
| EtOH | 289.9/330.3/490.0/525.5/579.1 | 288.4/330.6/477.8/505.2 | 554.4 | 537.0 | |
| DMSO | 294.2/336.8/488.0/522.6 | 292.0/334.2/477.2/509.0 | 547.0 | 536.0 | |


| Exp. | Cal. | ||
|---|---|---|---|
| $\lambda _{abs}^{\max }$a | Electronic Transition (contr.%) | λabsa/f | |
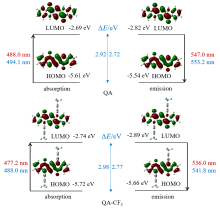
| QA | 488.0/522.6 | 81~>82 (99.11) | 494.1/0.0951 |
| 336.8 | 81~>84 (69.57) | 322.5/0.1091 | |
| 294.2 | 77~>82 (74.16) 81~>84 (20.90) | 291.9/1.4650 | |
| QA-CF3 | 477.2/509.0 | 153~>154 (98.96) | 488.0/0.1200 |
| 334.2 | 149~>154 (7.73) 153~>158 (86.68) | 331.5/0.1053 | |
| 292.0 | 147~>154 (15.78) 149~>154 (57.77) 153~>160 (18.94) | 293.4/0.8097 |
| Exp. | Cal. | ||
|---|---|---|---|
| $\lambda _{abs}^{\max }$a | Electronic Transition (contr.%) | λabsa/f | |
| QA | 488.0/522.6 | 81~>82 (99.11) | 494.1/0.0951 |
| 336.8 | 81~>84 (69.57) | 322.5/0.1091 | |
| 294.2 | 77~>82 (74.16) 81~>84 (20.90) | 291.9/1.4650 | |
| QA-CF3 | 477.2/509.0 | 153~>154 (98.96) | 488.0/0.1200 |
| 334.2 | 149~>154 (7.73) 153~>158 (86.68) | 331.5/0.1053 | |
| 292.0 | 147~>154 (15.78) 149~>154 (57.77) 153~>160 (18.94) | 293.4/0.8097 |

| [1] |
Song H. J.; Kim D. H.; Lee E. J.; Heo W. S.; Lee J. Y.; Moon D. K. Macromolecules 2012, 45, 7815.
|
| [2] |
Pham H. D.; Jain S. M.; Li M.; Manzhos S.; Feron K.; Pitchaimuthu S.; Liu Z. Y.; Motta N.; Wang H. X.; Durrant J. R.; Sonar P. J. Mater. Chem. A 2019, 7, 5315.
|
| [3] |
Głowacki E. D.; Irimia-Vladu M.; Kaltenbrunner M.; Gasiorowski J.; White M. S.; Monkowius U.; Romanazzi G.; Suranna G. P.; Mastrorilli P.; Sekitani T.; Bauer S.; Someya T.; Torsi L.; Sariciftci N. S. Adv. Mater. 2013, 25, 1563.
|
| [4] |
Salzillo T.; Rivalta A.; Castagnetti N.; D'Agostino S.; Masino M.; Grepioni F.; Venuti E.; Brillante A.; Girlando A. CrystEngComm 2019, 21, 3702.
doi: 10.1039/c9ce00070d |
| [5] |
Wang C. G.; Wang K.; Fu Q.; Zhang J. Y.; Ma D. G.; Wang Y. J. Mater. Chem. C 2013, 1, 410.
|
| [6] |
Wang C. G.; Wang S. P.; Chen W. P.; Zhang Z. L.; Zhang H. Y.; Wang Y. RSC Adv. 2016, 6, 19308.
|
| [7] |
Li J.; Lv X. J.; Zhang L.; Feng M. L.; Ouyang M.; Liu C. Y.; Xia M. N.; Zhang C. Dyes Pigm. 2022, 207, 110689.
|
| [8] |
Preda g.; Arico A.; Botta C.; Ravelli D.; Merli D.; Mattiello S.; Beverina L.; Pasini D. Org. Lett. 2023, 25, 6490.
|
| [9] |
Wang Y. F.; Li M.; Chen C. F. Acta Chim. Sinica 2023, 81, 588 (in Chinese).
|
|
(王银凤, 李猛, 陈传峰, 化学学报, 2023, 81, 588.)
doi: 10.6023/A23040153 |
|
| [10] |
Xie G. Z.; Brosius V.; Han J.; Rominge F.; Dreuw A.; Freudenberg J.; Bunz U. H. F. Chem. Eur. J. 2020, 26, 160.
|
| [11] |
(a) Mizuguchi J.; Senju T. J. Phys. Chem. B 2006, 110, 19154.
|
|
(b) Miyashita Y.; Yokoyama H.; Tanabe M.; Kasai H.; Nakanishi H.; Miyashita T. J. Photochem. Photobiol. A 2009, 201, 208.
|
|
| [12] |
Bera M. K.; Pal P.; Malik S. J. Mater. Chem. C 2020, 8, 788.
|
| [13] |
Zou Y.; Yuan T. Y.; Yao H. P.; Frazier D. J.; Stanton D. J.; Sue H. J.; Fang L. Org. Lett. 2015, 17, 3146.
|
| [14] |
Li M.; Xie W.; Cai X.; Peng X.; Liu K.; Gu Q.; Zhou J.; Qiu W.; Chen Z.; Gan Y.; Su S. J. Angew. Chem. Int. Ed. 2022, 61, e202209343
|
| [15] |
Chen S.; Chen Z. L.; Hu Q.; Meng Y. S.; Huang Y.; Tao P. F.; Lu L. R.; Huang G. B. Chin. J. Org. Chem. 2024, 44, 277 (in Chinese).
|
|
(陈珊, 陈志林, 胡琼, 蒙艳双, 黄悦, 陶萍芳, 卢丽如, 黄国保, 有机化学, 2024, 44, 277.)
doi: 10.6023/cjoc202306013 |
|
| [16] |
Yu C. S.; Wang H.; Min L. J.; Han L.; Shi J. J.; Liu X. H. Chin. J. Org. Chem. 2021, 41, 4498 (in Chinese).
|
|
(余陈升, 王翰, 闵莉静, 韩亮, 史建俊, 刘幸海, 有机化学, 2021, 41, 4498.)
doi: 10.6023/cjoc202106025 |
|
| [17] |
Kohn W.; Sham L. J. Phy. Rev. 1965, 140, A1133
|
| [18] |
Runge E.; Gross E. K. U. Phys. Rev. Lett. 1984, 52, 997.
|
| [19] |
Bauernschmitt R.; Ahlrichs R. Chem. Phys. Lett. 1996, 256, 454.
|
| [20] |
Casida M. E.; Jamorski C.; Casida K. C.; Salahub D. R. J. Chem. Phys. 1998, 108, 4439.
|
| [21] |
Stratmann R. E.; Scuseria G. E.; Frisch M. J. J. Chem. Phys. 1998, 109, 8218.
|
| [22] |
Furche F.; Ahlrichs R. J. Chem. Phys. 2002, 117, 7433.
|
| [23] |
Marques M. A. L.; Gross E. K. U. Annu. Rev. Phys. Chem. 2004, 55, 427.
pmid: 15117259 |
| [24] |
Chiba M.; Tsuneda T.; Hirao K. J. Chem. Phys. 2006, 124, 144106.
|
| [25] |
Casida M. E. J. Mol. Struct.: THEOCHEM 2009, 914, 3.
|
| [26] |
Becke A. D. J. Chem. Phys. 1993, 98, 5648.
|
| [27] |
Lee C.; Yang W. T.; Parr R. G. Phys. Rev. B 1988, 37, 785.
doi: 10.1103/physrevb.37.785 pmid: 9944570 |
| [28] |
Weigend F.; Ahlrichs R. Phys. Chem. Chem. Phys. 2005, 7, 3297.
|
| [29] |
Grimme S.; Ehrlich S.; Goerigk L. J. Comput. Chem. 2011, 32, 1456.
|
| [30] |
Grimme S.; Antony J.; Ehrlich S.; Krieg H. J. Chem. Phys. 2010, 132, 154104.
|
| [31] |
Amovilli C.; Barone V.; Cammi R.; Cancès E.; Cossi M.; Mennucci B.; Pomelli C. S.; Tomasi
|
| [32] |
Frisch M. J.; Trucks G. W.; Schlegel H. B.; Scuseria G. E.; Robb M. A.; Cheeseman J. R.; Scalmani G.; Barone V.; Mennucci B.; Petersson G. A.; Nakatsuji H.; Caricato M.; Li X.; Hratchian H. P.; Izmaylov A. F.; Bloino J.; Zheng G.; Sonnenberg J. L.; Hada M.; Ehara M.; Toyota K.; Fukuda R.; Hasegawa J.; Ishida M.; Nakajima T.; Honda Y.; Kitao O.; Nakai H.; Vreven T.; Montgomery J. A.; Jr.; Peralta J. E.; Ogliaro F.; Bearpark M.; Heyd J. J.; Brothers E.; Kudin K. N.; Staroverov V. N.; Kobayashi R.; Normand J.; Raghavachari K.; Rendell A.; Burant J. C.; Iyengar S. S.; Tomasi J.; Cossi M.; Rega N.; Millam J. M.; Klene M.; Knox J. E.; Cross J. B.; Bakken V.; Adamo C.; Jaramillo J.; Gomperts R.; Stratmann R. E.; Yazyev O.; Austin A. J.; Cammi R.; Pomelli C.; Ochterski J. W.; Martin R. L.; Morokuma K.; Zakrzewski V. G.; Voth G. A.; Salvador P.; Dannenberg J. J.; Dapprich S.; Daniels A. D.; Farkas Ö.; Foresman J. B.; Ortiz J. V.; Cioslowski J.; Fox D. J. Gaussian 09, Revision D.01; Gaussian, Inc., Wallingford CT, 2009.
|
| [33] |
Dennington R.; Keith T. A.; Millam J. M. GaussView, Version 6, Semichem Inc., Shawnee Mission, KS, 2016.
|
| [34] |
Lu T.; Chen F. J. Comput. Chem. 2012, 33, 580.
|
| [35] |
Humphrey W.; Dalke A.; Schulten K. J. Mol. Graph. 1996, 14, 33.
|
| [36] |
Neese F. WIREs Comput. Mol. Sci. 2012, 2, 73.
|
| [37] |
Neese F.; Wennmohs F.; Becker U.; Riplinger C. J. Chem. Phys. 2020, 152, 224108.
|
| [38] |
Neese F. WIREs Comput. Mol. Sci. 2018, 8, e1327
|
| [39] |
Neese F. WIREs Comput. Mol. Sci. 2022, 12, e1606
|
| [40] |
Neese F.; Wennmohs F.; Hansen A.; Becker U. Chem. Phys. 2009, 356, 98.
|
| [41] |
Izsák R.; Neese F. J. Chem. Phys. 2011, 135, 144105.
|
| [42] |
Izsák R.; Neese F.; Klopper W. J. Chem. Phys. 2013, 139, 094111.
|
| [43] |
Helmich-Paris B.; de Souza B.; Neese F.; Izsák R. J. Chem. Phys. 2021, 155, 104109.
|
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||