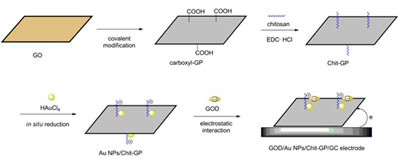
Based on the covalent modification and in-situ reduction, chitosan and gold nanoparticles were integrated with graphene to form a novel gold nanoparticles/chitosan-graphene (AuNPs/Chit-GP) nanocomposite. The process of the fabrication of AuNPs/Chit-GP nanocomposite consisted of the following steps. Firstly, carboxyl-GP was prepared based on the reduction and modification of graphene oxide (GO). Thereafter, chitosan was covalently functionalized onto graphene nanosheet to fabricate Chit-GP. The AuNPs/Chit-GP nanocomposite was finally synthesized by the in-situ reduction of HAuCl4 in the presence of Chit-GP. Fourier transform infrared (FT-IR) spectra, ultraviolet-visible (UV-vis) absorption spectra, transmission electron microscopy (TEM) and X-ray diffraction (XRD) were utilized to characterize structure and morphology of the as synthesized nanocomposite. Because of the surface functionalization of chitosan, the composite exhibited positive charge. In that case, the negatively charged glucose oxidase (GOD) could further immobilize onto AuNPs/Chit-GP via electrostatic interaction under mild experimental condition. With the advantages of graphene, Au nanoparticles and chitosan, AuNPs/Chit-GP can offer a conductive and favorable microenvironment for the immobilized GOD to achieve direct electrochemistry. The direct electron transfer (DET) reaction of the immoblized GOD was studied by cyclic voltammetry in 0.1 mol/L phosphate buffer solution (PBS, pH 7.4). A pair of well-defined, quasi-reversible redox peaks of GOD were obtained at GOD/AuNPs/Chit-GP/GC modified electrode, with a formal potential (vs. Ag/AgCl) being -0.44 V. Moreover, the as fabricated GOD/AuNPs/Chit-GP/GC modified electrode exhibited excellent catalytic performance towards glucose.
The electrocatalytic response of GOD/AuNPs/Chit-GP/GC modified electrode altered linearly with the glucose concentration ranging from 2.1 to 5.7 μmol/L. The detection limit and the sensitivity of the enzyme electrode were 0.7 μmol/L (S/N=3) and 79.71 mA·cm-2·mM-1, respectively. Because of the biocompability of the AuNPs/Chit-GP, GOD/AuNPs/Chit-GP/GC modified electrode also exhibited acceptable reproducibility and excellent stability. Therefore, such a novel nanocomposite composed of metal nanoparticles, biocompatible macromolecules and graphene provides an efficient platform for the development of mediator-free electrochemical biosensors.
[1] Wang, Y.; Li, Z. H.; Li, J. H.; Lin, Y. H. Trends Biotechnol. 2011, 29, 205.
[2] Liu, Y.; Wang, M.; Zhao, F.; Xu, Z.; Dong, S. J. Biosens. Bioelectron. 2005, 21, 984.
[3] Zeng, Q.; Cheng, J. S.; Tang, L. H.; Liu, X. F.; Liu, Y. Z.; Li, J. H.; Jiang, J. H. Adv. Funct. Mater. 2010, 20, 3366.
[4] Zhang, L.; Zhang, Q.; Li, J. H. Adv. Funct. Mater. 2007, 17, 1958.
[5] Zhang, Y.; Zheng, J. b. Acta Chim. Sinica 2011, 69, 1903. (张亚, 郑建斌, 化学学报, 2011, 69, 1903.)
[6] Wang, Y.; Li, Z.; Hu, D.; Lin, C. T.; Li, J. H.; Lin, Y. H. J. Am. Chem. Soc. 2010, 132, 9274.
[7] Tang, L. H.; Wang, Y.; Li, Y. M.; Feng, H. B.; Lu, J.; Li, J. H. Adv. Funct. Mater. 2009, 19, 782.
[8] Wang, Y.; Shao, Y. Y.; Dean W. M.; Lin, Y. H.; Li, J. H. ACS Nano 2010, 4, 1790.
[9] Liu, S.; Tian, J. Q.; Luo, Y. L.; Lu, W. B.; Sun, X. P. Biosens. Bioelectron. 2011, 26, 4491.
[10] Jiang, H. J.; Zhao, Y.; Yang, H.; Aking, D. L. Mater. Chem. Phys. 2009, 114, 879.
[11] Chen, D.; Tang, L. H.; Li, J. H. Chem. Soc. Rev. 2010, 39, 3157.
[12] Zhang, H.; Lv, X. J.; Li, Y. M.; Wang, Y.; Li, J. H. ACS Nano 2009, 4, 380.
[13] Jin, Y.; Chen, H.-Y.; Chen, M.-H.; Liu, N.; Li, Q.-W. Acta Phys.-Chim. Sinica 2012, 28, 609. (靳瑜, 陈宏源, 陈名海, 刘宁, 李清文, 物理化学学报, 2012, 28, 609.)
[14] Wu, J. F.; Xu, M. Q.; Zhao, G. C. Electrochem. Commun. 2010, 12, 175.
[15] Swoboda, B. E. P.; Massey, V. J. Biol. Chem. 1965, 240, 2209.
[16] Leskovac, V.; Trivić, S.; Wohlfahrt, G.; Kandrac, J.; Pericin, D. Int. J. Biochem. Cell B 2005, 37, 731.
[17] Dupont, J.; Spencer, J. Angew. Chem. Int. Ed. 2004, 43, 5296.
[18] Shan, C.; Yang, H.; Song, J.; Han, D.; Ivaska, A.; Niu, L. Anal. Chem. 2009, 81, 2378.
[19] Liu, Q.; Lu, X. B.; Li, J.; Yao, X.; Li, J. H. Biosens. Bioelectron. 2007, 22, 3203.
[20] Xiao, C.; Chu, X.; Wu, B.; Pang, H.; Zhang, X.; Chen, J. Talanta 2010, 80, 1719.
[21] Liu, S. Q.; Ju, H. X. Biosens. Bioelectron. 2003, 19, 177.
[22] Kang, X. H.; Wang, J.; Wu, H.; Aksay, I. A.; Liu, J.; Lin, Y. H. Biosens. Bioelectron. 2009, 25, 901.
[23] Hummers, W. S.; Offeman, R. E. J. Am. Chem. Soc. 1958, 80, 1339. Lomeda, J. R.; Doyle, C. D.; Kosynkin, D. V.; Hwang, W. F.; Tour, J. M. J. Am. Chem. Soc. 2008, 130, 16201.


