

Synthesis and Supercapacitor Property of Three-dimensional Graphene/Ni-Al Layered Double Hydroxide Composite
Received date: 2013-01-12
Online published: 2013-03-26
Supported by
Project supported by the National Natural Science Foundation of China (No. 21176101) and the Ministry of Education to Independent Research Program (No. JUSRP51314B).
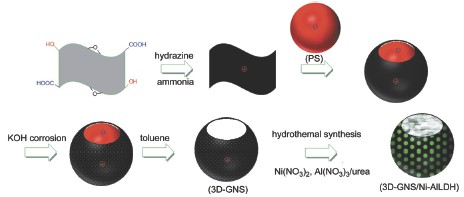
Graphite oxide and polystyrene colloidal microsphere (PS) were dispersed in deionized water with the help of ultrasonic wave to form a stable dispersion. The ammonia and hydrazine were seperately added to the dispersion to reduce graphene oxide and form the PS wrapped with graphene nanosheet. During the process, graphite oxide was chemically reduced by hydrazine in the presence of ammonia to produce positively charged reduced graphite oxide, then the PS colloidal particles negtively charged were wrapped by the graphene nanosheets to form PS/graphene microspheres due to the electrostatic interactions between them. To obtain three-dimensional macroporous graphene nanosheets (3D-GNS), it was orderly treated by the alkali corrosion in a 6 mol·L-1 potassium hydroxide solution and remove of the PS in a toluene. The as-prepared 3D-GNS was well dispersed in deionized water by means of ultrasonic wave and then hydrothermal synthesis method was used to prepare 3D graphene/nickel-aluminium layered double-hydroxide (3D-GNS/Ni-Al LDH) nanocomposite in a Teflon-lined stainless steel autoclave at 100 ℃ for 24 h, in which nickel nitrate, aluminum nitrate and urea were employed as nickel, aluminium and base resources. In this study, IR spectrum, Raman spectroscopy, X-ray diffraction, scanning electron microscopy, transmission electron microscopy and galvanostatic charge-discharge measurement were used to investigate the structure, morpholoy and electrochemical property of the nanocomposite respectively. It was found that the graphite oxide was effectively reduced into the graphene with a 3D micropore structure. Ni-Al LDH nanoflakes were well dispersed in and out of the wall of 3D-GNS. Moreover, electrochemical performance of the 3D-GNS/Ni-Al LDH composite was investigated as supercapacitor electrode materials. A 1054.8 F·g-1 of the specific capacitance was found at the current density of 1 A·g-1. When the current density increased up to 8 A·g-1, the specific capacitance remains 628.1 F·g-1. The value was above 97% of capacitance retention after 1000 cycles, indicating that the composite is of excellent electrochemical performance.
Yan Lin , Kong Hui , Li Zaijun . Synthesis and Supercapacitor Property of Three-dimensional Graphene/Ni-Al Layered Double Hydroxide Composite[J]. Acta Chimica Sinica, 2013 , 71(05) : 822 -828 . DOI: 10.6023/A13010056
/
| 〈 |
|
〉 |