

Biocompatible Phospholipid Modified Graphene Nanocomposite for Direct Electrochemistry of Redox Enzyme
Received date: 2013-08-30
Online published: 2013-11-14
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21235004, 21128005, 51273087, 21071070, 21203126, 21005046, 51203072, 20901035), the Financial Supports from the Program for Liaoning Innovative Research Team in University (No. LT2011001), the Natural Science Foundation of Liaoning Province (Nos. 201202088, LJQ2013112), the Research Fund for the Doctoral Program of Liaoning Province (No. 20131042), the Foundation for Young Scholars of Liaoning University (Nos. 2012LDQN08, 2012LDQN07), College students' innovative entrepreneurial training program (No. X201210140036) and the Foundation of 211 Project for Innovative Talents Training, Liaoning University.
A novel lipid based carbonaceous nanocomposite, 1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-(1'-rac-glycerol) (sodium salt) (POPG) modified graphene (GP) (POPG-GP), was designed and synthesized by a non-covalent method. The nanocomposite was endowed with excellent properties of the two independent components, such as the biocompatibility of POPG and the outstanding electric properties of graphene. Fourier transform infrared (FT-IR) spectra, ultraviolet-visible (UV-vis) absorption spectra, transmission electron microscopy (TEM) were utilized to characterize the structure, morphology and surface property of the as synthesized POPG-GP. It has been found that the modification of POPG on GP could not only assist the dispersion of graphene in aqueous solution, but also endow it with negatively charged, which was favorable for the further immobilization of model enzyme via self-assembly. Based on the electrostatic interaction, the positively charged horseradish peroxide (HRP) could be immobilized onto the surface of POPG-GP to form HRP/POPG-GP/GC electrode. UV-vis and FT-IR spectroscopies were used to monitor the assembly process and demonstrated that HRP had been immobilized without denaturation. The HRP/POPG-GP/GC electrode could commendably realize the direct electron transfer (DET) between electrode and redox enzyme with good electrochemical performance. Moreover, such modified electrode also showed good electrocatalytic response toward the detection of H2O2 with high sensitivity, wide linear range, excellent stability and reproducibility. The linear response range for the HRP/POPG-GP/GC was 3.5~210 μmol/L (R=0.999). The detection limit and the sensitivity of the HRP/POPG-GP/GC electrode was calculated to be 1.17 μmol/L (S/N=3) and 356.6 mA·cm-2·M-1, respectively. The apparent Michaelis-Menten constant Km was estimated to be 0.45 mmol/L, indicating a high affinity of HRP to H2O2 on POPG-GP. The experiment results demonstrated that POPG-GP not only provided a biocompatible microenvironment for the immobilized HRP, but also supplied a necessary pathway for its direct electron transfer. Therefore, such biocompatible nanocomposite had potential applications in the field of biosensors.
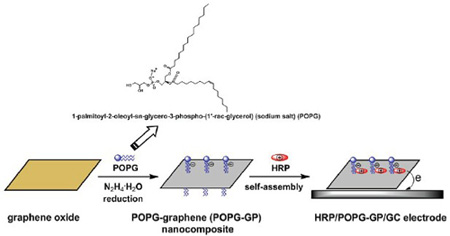
Key words: graphene; phospholipid; nanocomposite; horseradish peroxide; direct electrochemistry
Zhang Qian , Wu Shuyao , Zhang Ling , Mao Hui , Liu Daliang , Liu Yang , Zeng Xiangqun , Song Ximing , Li Jinghong . Biocompatible Phospholipid Modified Graphene Nanocomposite for Direct Electrochemistry of Redox Enzyme[J]. Acta Chimica Sinica, 2014 , 72(3) : 388 -394 . DOI: 10.6023/A13080911
[1] Kang, X.; Wang, J.; Wu, H.; Aksay, I. A.; Liu, J.; Lin, Y. Biosens. Bioelectron. 2009, 25, 901.
[2] Liu, Y.; Wang, M.; Zhao, F.; Xu, Z.; Dong, S. Biosens. Bioelectron. 2005, 21, 984.
[3] Chen, H.; Dong, S. Biosens. Bioelectron. 2007, 22, 1811.
[4] Lu, X.; Hu, J.; Yao, X.; Wang, Z.; Li, J. Biomacromolecules 2006, 7, 975.
[5] Dai, Z.; Ju, H. Acta Phys.-Chim. Sin. 2004, 20, 1262. (戴志晖, 鞠熀先, 物理化学学报, 2004, 20, 1262.)
[6] Lu, X.; Zhang, Q.; Zhang, L.; Li, J. Electrochem. Commun. 2006, 8, 874.
[7] Zhang, Q.; Wu, S.; Zhang, L.; Lu, J.; Verproot, F.; Liu, Y.; Xing, Z.; Li, J.; Song, X.-M. Biosens. Bioelectron. 2011, 26, 2632.
[8] Cai, C.; Chen, J. Acta Chim. Sinica 2004, 62, 335. (蔡称心, 陈静, 化学学报, 2004, 62, 335.)
[9] Novoselov, K. S.; Geim, A. K.; Morozov, S. V.; Jiang, D.; Katsnelson, M. I.; Grigorieva, I. V.; Dubonos, S. V.; Firsov, A. A. Nature 2005, 438, 197.
[10] Li, D.; Muller, M. B.; Gilje, S.; Kaner, R. B.; Wallace, G. G. Nat. Nanotechnol. 2008, 3, 101.
[11] Zhang, Q.; Yang, S.; Zhang, J.; Zhang, L.; Kang, P.; Li, J.; Xu, J.; Zhou, H.; Song, X.-M. Nanotechnology 2011, 22, 494010.
[12] Zhang, Q.; Wu, S.; He, M.; Zhang, L.; Liu, Y.; Li, J.; Song, X.-M. Acta Chim. Sinica 2012, 70, 2213. (张谦, 吴抒遥, 何茂伟, 张玲, 刘洋, 李景虹, 宋溪明, 化学学报, 2012, 70, 2213.)
[13] Niyogi, S.; Bekyarova, E.; Itkis, M. E.; McWilliams, J. L.; Hamon, M. A.; Haddon, R. C. J. Am. Chem. Soc. 2006, 128, 7720.
[14] Yang, H.; Zhang, Q.; Shan, C.; Li, F.; Han, D.; Niu, L. Langmuir 2010, 26, 6708.
[15] Dayani, Y.; Malmstadt, N. Langmuir 2012, 28, 8174.
[16] Hummers, W. S.; Offeman, R. E. J. Am. Chem. Soc. 1958, 80, 1339.
[17] Wang, Z.; Li, M.; Su, P.; Zhang, Y.; Shen, Y.; Han, D.; Ivaska, A.; Niu, L. Electrochem. Commun. 2008, 10, 306.
[18] Li, J.; Dong, S. J. Electroanal. Chem. 1997, 431, 19.
[19] Zhang, L.; Zhang, Q.; Lu, X.; Li, J. Biosens. Bioelectron. 2007, 23, 102.
[20] Bond, A. M.; Braun, R. D. J. Electrochem. Soc. 1980, 127, 528C.
[21] Zhang, Q.; Qiao, Y.; Hao, F.; Zhang, L.; Wu, S.; Li, Y.; Li, J.; Song, X. M. Chem. Eur. J. 2010, 16, 8133.
[22] Mohammed, E.; Ignacio, N.-R.; Manuel, D.; Maria, P. H.-A.; Dolores, B.-M.; Jose, L. H.-H. C. Electrochim. Acta 2008, 53, 7131.
[23] Kamin, R. A.; Wilson, G. S. Anal. Chem. 1980, 52, 1198.
[24] Zhao, X.; Mai, Z.; Kang, X.; Zou, X. Biosens. Bioelectron. 2008, 23, 1032.
[25] Zhou, K.; Zhu, Y.; Yang, X.; Luo, J.; Li, C.; Luan, S. Electrochim. Acta 2010, 55, 3055.
/
| 〈 |
|
〉 |