

One-pot Suzuki-Heck Reaction to Construct Luminescent Microporous Organic Polymers Based on 4-Vinylphenylbororic Acid
Received date: 2015-01-29
Online published: 2015-03-27
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 91122029 and 21471064) and the State Basic Project of China (No. 2011CB808703).
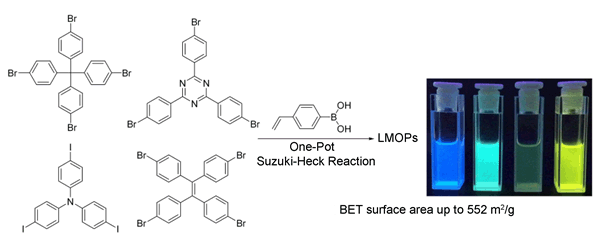
In recent years the increasing needs of applications have promoted the evolution of porous organic materials (POPs), which can be constructed by copolymerization of organic monomers based on topology chemistry. The advantages of these materials such as excellent physical and chemical stability, low framework density, various structure features, have made them good candidates in gas storage and separation, catalysis, sensors and so on. In this work, a one-pot synthetic strategy has been developed to construct a series of luminescent microporous organic polymers (LMOPs) by the palladium catalyzed Suzuki-Heck cascade coupling reactions of 4-vinylphenylboronic acid with aromatic halides, such as tetrakis(4-bromophenyl)-methane (TBPM), tris(4-iodophenyl)amine (TIPA), 1,1,2,2-tetrakis(4-bromophenyl)ethene (TBPE) and 2,4,6-tris(4-bromophenyl)-1,3,5-triazine (TBPT). Through the optimized conditions, the Pd(OAc)2/(o-tol)3P catalyst exhibits the highest efficiency in such a system. The FTIR measurements combined with the solid state 13C NMR are employed to confirm the existence of the resultant functional groups, which further proves the success of such polymerization. The resultant materials show porous features with the N2 adsorption-desorption measurements, with the BET surface areas ranging from 274 to 552 m2·g-1. Furthermore, with the incorporation of vinyl groups, the polymers exhibit visible luminescent feature from blue to yellow. Considering the emission behaviours of these polymers, the selective quenching toward picric acid is studied with the comparison of other nitroaromatic analytes. The results show that LMOP-11 has the highest sensing ability among these polymers. LMOP-12 shows excellent reusable ability towards picric acid. Such a one-pot method for the preparation of aromatic halides with 4-vinylboronic acid provides a simple and efficient synthetic mean to produce luminescent microporous organic framework that could be used in the selective sensing of explosives.
Sun Libo , Liang Zhiqiang , Yu Jihong . One-pot Suzuki-Heck Reaction to Construct Luminescent Microporous Organic Polymers Based on 4-Vinylphenylbororic Acid[J]. Acta Chimica Sinica, 2015 , 73(6) : 611 -616 . DOI: 10.6023/A15010077
[1] (a) Dawson, R.; Cooper, A. I.; Adams, D. J. Prog. Polym. Sci. 2012, 37, 530;
(b) Wu, D.; Xu, F.; Sun, B.; Fu, R.; He, H.; Matyjaszewski, K. Chem. Rev. 2012, 112, 3959;
(c) Xu, Y. Jin, S.; Xu, H.; Nagai, A.; Jiang, D. Chem. Soc. Rev. 2013, 42, 8012.
[2] Yu, J. H.; Yan, W. F. Chemistry of Nanoporous Materials, Science Press, Beijing, 2013. (于吉红, 闫文付主编, 纳米孔材料化学, 科学出版社, 北京, 2013.)
[3] (a) Kaur, P.; Hupp, J. T.; Nguyen, S. T. ACS Catal. 2011, 1, 819;
(b) Zhang Y.; Riduan, S. N. Chem. Soc. Rev. 2012, 41, 2083;
(c) Liang, L.-Y.; Li, B.-Y.; Chen, B.; Zhou, B.; Chen, K.-P.; Tan, B.-E. Polym. Bull. 2008, 10, 6. (梁丽芸, 李步怡, 陈冰, 周壁, 陈可平, 谭必恩, 高分子通报, 2008, 10, 6);
(d) Han, S. S.; Furukawa, H.; Yaghi, O. M.; Goddard, W. A. J. Am. Chem. Soc. 2008, 130, 11580.
[4] (a) Côté, A. P.; Benin, A. I.; Ockwig, N. W.; Matzger, A. J.; O'Keeffe M.; Yaghi, O. M. Science 2005, 310, 1166;
(b) El-Kaderi, H. M.; Hunt, J. R.; Mendoza-Cortés, J. L.; Côté, A. P.; Taylor, R. E.; O'Keeffe M.; Yaghi, O. M. Science 2007, 316, 268.
[5] (a) Feng, X.; Ding X.; Jiang, D. Chem. Soc. Rev. 2012, 41, 6010;
(b) Ding S.-Y.; Wang, W. Chem. Soc. Rev. 2013, 42, 548.
[6] (a) Uribe-Romo, F. J.; Hunt, J. R.; Furukawa, H.; Klock, C.; O'Keeffe, M.; Yaghi, O. M. J. Am. Chem. Soc. 2009, 131, 4570;
(b) Jin, Y.; Zhu, Y.; Zhang, W. CrystEngComm 2013, 15, 1484.
[7] (a) Weber, J.; Antonietti, M.; Thomas, A. Macromolecules 2008, 41, 2880;
(b) Wang, Z.; Zhang, B.; Yu, H.; Sun, L.; Jiao, C.; Liu, W. Chem. Commun. 2010, 46, 7730.
[8] (a) McKeown, N. B.; Budd, P. M. Chem. Soc. Rev. 2006, 35, 675;
(b) McKeown, N. B.; Gahnem, B.; Msayib, K. J.; Budd, P. M.; Tattershall, C. E.; Mahmood, K.; Tan, S.; Book, D.; Langmi H. W.; Walton, A. Angew. Chem., Int. Ed. 2006, 45, 1804.
[9] (a) Kou, Y.; Xu, Y.; Guo, Z.; Jiang, D. Angew. Chem., Int. Ed. 2011, 50, 8753;
(b) Guo J.; Xu, Y. Jin, S.; Chen, L.; Kaji, T.; Honsho, Y.; Addicoat, M. A.; Kim, J.; Saeki, A.; Ihee, H.; Seki, S.; Irle, S.; Hiramoto1, M.; Gao, J.; Jiang, D. Nat. Commun. 2013, 4, 2736.
[10] Rabbani M. G.; El-Kaderi, H. M. Chem. Mater. 2011, 23, 1650.
[11] Macintyre, F. S.; Sherrington, D. C.; Tetley, L. Macromolecules 2006, 39, 5381.
[12] (a) Li, B.; Gong, R.; Wang, W.; Huang, X.; Zhang, W.; Li, H.; Hu C.; Tan, B. Macromolecules 2011, 44, 2410;
(b) Luo, Y.; Li, B.; Wang, W.; Wu, K.; Tan, B. Adv. Mater. 2012, 24, 5703.
[13] (a) Schmidt, J.; Weber, J.; Epping, J. D.; Antonietti, M.; Thomas, A. Adv. Mater. 2009, 21, 702;
(b) Chen, Q.; Luo, M.; Hammershøj, P.; Zhou, D.; Han, Y.; Laursen, B. W.; Yan, C.-G.; Han, B.-H. J. Am. Chem. Soc. 2012, 134, 6084.
[14] (a) Kuhn, P.; Antonietti, M.; Thomas, A. Angew. Chem., Int. Ed. 2008, 47, 3450;
(b) Wang, W.; Yan, Z.-J.; Yuan, Y.; Sun, F.-X.; Zhao, M.; Ren, H.; Zhu, G.-S. Acta Chim. Sinica 2014, 72, 557. (王维, 闫卓君, 元野, 孙福兴, 赵明, 任浩, 朱广山, 化学学报, 2014, 72, 557.)
[15] (a) Yuan, S.; Kirklin, S.; Dorney, B.; Liu, D.-J.; Yu, L. Macromolecules 2009, 42, 1554;
(b) Wang, C. A.; Zhang, Z. K.; Yue, T.; Sun, Y. L.; Wang, L.; Wang, W. D.; Zhang, Y.; Liu C.; Wang, W. Chem.-Eur. J. 2012, 18, 6718.
[16] Ben, T.; Ren, H.; Ma, S.; Cao, D.; Lan, J.; Jing, X.; Wang, W.; Xu, J.; Deng, F.; Simmons, J. M.; Qui, S.; Zhu, G. Angew. Chem., Int. Ed. 2009, 48, 9457.
[17] Weber, J.; Thomas, A. J. Am. Chem. Soc. 2008, 130, 6334.
[18] Jiang, J. X.; Su, F.; Trewin, A.; Wood, C. D.; Campbell, N. L.; Niu, H.; Dickinson, C.; Ganin, A. Y.; Rosseinsky, M. J.; Khimyak, Y. Z.; Cooper, A. I. Angew. Chem., Int. Ed. 2007, 46, 8574.
[19] Holst, J. R.; Stöckel, E.; Adams, D. J.; Cooper, A. I. Macromolecules 2010, 43, 8531.
[20] (a) Sun, L.; Liang, Z.; Yu, J.; Xu, R. Polym. Chem. 2013, 4, 1932;
(b) Sun, L.; Liang, Z.; Yu, J. Polym. Chem. 2015, 6, 917.
[21] Sun, L.; Zou, Y.; Liang, Z.; Yu, J.; Xu, R. Polym. Chem. 2014, 5, 471.
[22] (a) Traiphol, R.; Sanguansat, P.; Srikhirin, T.; Kerdcharoen, T.; Osotchan, T. Macromolecules 2006, 39, 1165;
(b) Song, I. Y.; Kim, J.; Im, M. J.; Moon, B. J.; Park, T. Macromolecules 2012, 45, 5058.
[23] Nagarkar, S. S.; Joarder, B.; Chaudhari, A. K.; Mukherjee, S.; Ghosh, S. K. Angew. Chem., Int. Ed. 2013, 52, 2881.
[24] Rathore, R.; Burns, C. L.; Guzei, I. A. J. Org. Chem. 2004, 69, 1524.
[25] Feng, J.; Zhang, C.; Li, Y.; Yang, M. J. Appl. Polym. Sci. 2011, 121, 217.
[26] (a) Jana, D.; Ghorai, B. K. Tetrahedron Lett. 2012, 53, 6838;
(b) Wu, W.; Ye, S.; Yu, G.; Liu, Y.; Qin, J.; Li, Z. Macromol. Rapid Commun. 2012, 33, 164.
[27] Ren, S.; Dawson, R.; Laybourn, A.; Jiang, J.-X.; Khimyak, Y.; Adams, D. J.; Cooper, A. I. Polym. Chem. 2012, 3, 928.
/
| 〈 |
|
〉 |