

Computational Insights into the Diels-Alder-alike Reactions of 1-Iodo-2-Lithio-o-Carborane with Fulvenes
Received date: 2017-08-03
Online published: 2017-10-20
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21363028, 21763033, 21373030) and Innovative Training Program for College Students in Yunnan Province.
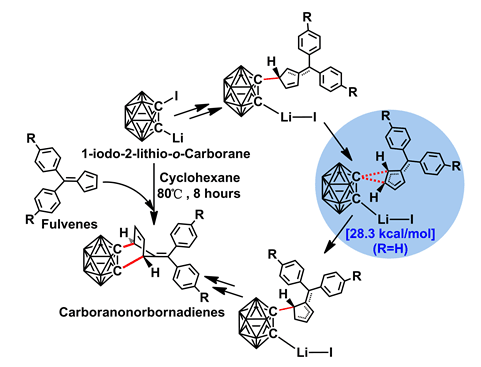
Density functional theory (DFT) calculations at the B3LYP level, combining with the double-ζ valence polarized (DZVP) all-electron basis set as embeded in Gaussian 09 Program, were carried out to investigate the reaction mechanisms and substituent effect of Diels-Alder-alike reactions between 1-iodo-2-lithio-o-carborane and fulvenes. For maximum analogy with experimental conditions, all calculations were carried out in cyclohexane solution by using the IDSCRF solvent model, and all energies reported here had been adjusted adaptive to experimental temperature (353 K). According to presently obtained results, this reaction needs to go through a four-step process successively before the final carboranonorbornadiene products are formed. These four steps include forming carboryne intermediate by release of LiI fragment, interaction of carboryne with fulvenes, 1,2-σ migration of carboranyl, and the cycloaddition process. Among all four steps mentioned above, the 1,2-σ migration of carboranyl is predicted to be the rate-determining step (RDS), features an activation free energy barrier of 28.3 kcal•mol-1 under experimental temperature of 353 K. A half-life of 8.7 h converted from the RDS activation free energy barrier coincides well with corresponding 56% isolated yield of carboranonorbornadiene after reacted 8 h. The LiI fragment is found to be vital in stabilizing most stationary points and driving the reaction ahead. The reaction mechanisms change little when the 4-H substituents on diphenylfulvenes (denoted reaction a) is replaced by 4-Me groups (denoted reaction b), but the corresponding RDS activation free energy barrier increased by 2.8 kcal•mol-1 (from 28.3 to 31.1 kcal•mol-1), transferring to a decrease in reaction rate of ca. 50 times. The obvious slower reaction rate predicted in reaction b than in reaction a gives out correct trends with an experimental yield reduction of carboranonorbornadienes from 56% to 42%, and verifies the rationality of B3LYP results in these carboranyl-involved Diels-Alder-alike reactions. Natural bond orbital (NBO) analysis about corresponding reactants and stationary points shows similar electronic characteristics of this reaction with normal-electron-demand Diels-Alder (NEDDA) reactions, i.e., the fulvenes act as electron donator when react with carboryne intermediate.
Mu Weihua , Ma Yao , Fang Decai , Wang Rong , Zhang Haina . Computational Insights into the Diels-Alder-alike Reactions of 1-Iodo-2-Lithio-o-Carborane with Fulvenes[J]. Acta Chimica Sinica, 2018 , 76(1) : 55 -61 . DOI: 10.6023/A17080357
[1] Li, Y.; Li, W.; Zhang, J. Chem. Eur. J. 2017, 23, 467.
[2] Pellissier, H. Chem. Rev. 2016, 116, 14868.
[3] Wei, L.; Wang, C.-J. Chem. Commun. 2015, 51, 15374.
[4] Ai, W.; Liao, D.; Lei, X. Chin. J. Org. Chem. 2015, 35, 1615(in Chinese). (艾文英, 廖道红, 雷晓光, 有机化学, 2015, 35, 1615.)
[5] Liu, W.; Wu, Y.; Li, L.; Li, X. Chin. J. Org. Chem. 2016, 36, 1501(in Chinese). (刘文香, 吴宇强, 李灵芝, 李霞, 有机化学, 2016, 36, 1501.)
[6] Cao, M.-H.; Green, N. J.; Xu, S.-Z. Org. Biomol. Chem. 2017, 15, 3105.
[7] Xie, M.; Lin, L.; Feng, X. Chem. Rec. 2017, 17, 1.
[8] Fang, D.; Chen, Y. Acta Chim. Sinica 2014, 72, 253(in Chinese). (方德彩, 陈彦梅, 化学学报, 2014, 72, 253.)
[9] Ran, Y.; Tang, M.; Wang, Y.; Wang, Y.; Zhang, X.; Zhu, Y.; Wei, D.; Zhang, W. Tetrahedron 2016, 72, 5295.
[10] Musavi, S. M.; Amani, J.; Omidian, N. Tetrahedron 2014, 70, 708.
[11] Li, Y.; Fang, D.-C. Phys. Chem. Chem. Phys. 2014, 16, 15224.
[12] Tang, M.; Wu, Y.; Liu, Y.; Cai, M.; Xia, F.; Liu, S.; Hu, W. Acta Chim. Sinica 2016, 74, 54(in Chinese). (唐敏, 吴永, 刘源, 蔡茂强, 夏飞, 刘顺英, 胡文浩, 化学学报, 2016, 74, 54.)
[13] Yang, Y.-F.; Liang, Y.; Liu, F.; Houk, K. N. J. Am. Chem. Soc. 2016, 138, 1660.
[14] Duan, A.; Yu, P.; Liu, F.; Qiu, H.; Gu, F. L.; Doyle, M. P.; Houk, K. N. J. Am. Chem. Soc. 2017, 139, 2766.
[15] Yang, Y.; Liu, Q.; Zhang, L.; Yu, H.; Dang, Z. Organometallics 2017, 36, 687.
[16] Li, Y.; Du, S. RSC Adv. 2016, 6, 84177.
[17] Diamond, O. J.; Marder, T. B. Org. Chem. Front. 2017, 4, 891.
[18] Ess, D. H.; Jones, G. O.; Houk, K. N. Adv. Synth. Catal. 2006, 348, 2337.
[19] Yu, P.; Yang, Z.; Liang, Y.; Hong, X.; Li, Y.; Houk, K. N. J. Am. Chem. Soc. 2016, 138, 8247.
[20] Yu, P.; Li, W.; Houk, K. N. J. Org. Chem. 2017, 82, 6398.
[21] Pellissier, H. Adv. Synth. Catal. 2016, 358, 2194.
[22] Mojica, M.; Méndez, F.; Alonso, J. A. Molecules 2016, 21, 200.
[23] Oliveira, B. L.; Guo, Z.; Bernardes, G. J. L. Chem. Soc. Rev. 2017, 46, 4895.
[24] Fell, J. S.; Lopez, S. A.; Higginson, C. J.; Finn, M. G.; Houk, K. N. Org. Lett. 2017, 19, 4504.
[25] Liu, F.; Liang, Y.; Houk, K. N. J. Am. Chem. Soc. 2014, 136, 11483.
[26] Levandowski, B. J.; Zou, L.; Houk, K. N. J. Comput. Chem. 2016, 37, 117.
[27] Zhang, J.; Qiu, Z.; Xu, P.-F.; Xie, Z. ChemPlusChem 2014, 79, 1044.
[28] Tang, C.; Xie, Z. Angew. Chem., Int. Ed. 2015, 54, 7662.
[29]
(a) Lyu, H.; Quan, Y.; Xie, Z. Angew. Chem., Int. Ed. 2015, 54, 10623;
(b) Lyu, H.; Quan, Y.; Xie, Z. J. Am. Chem. Soc. 2016, 138, 12727.
[30] Lee, C.; Yang, W.; Parr, R. G. Phys. Rev. B 1988, 37, 785.
[31] Becke, A. D. J. Chem. Phys. 1993, 98, 5648.
[32] Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. A.; Nakatsuji, H.; Caricato, M.; Li, X.; Hratchian, H. P.; Izmaylov, A. F.; Bloino, J.; Zheng, G.; Sonnenberg, J. L.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Montgomery, J. A., Jr.; Peralta, J. E.; Ogliaro, F.; Bearpark, M.; Heyd, J. J.; Brothers, E.; Kudin, K. N.; Staroverov, V. N.; Keith, T.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A.; Burant, J. C.; Iyengar, S. S.; Tomasi, J.; Cossi, M.; Rega, N.; Millam, J. M.; Klene, M.; Knox, J. E.; Cross, J. B.; Bakken, V.; Adamo, C.; Jaramillo, J.; Gomperts, R.; Stratmann, R. E.; Yazyev, O.; Austin, A. J.; Cammi, R.; Pomelli, C.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Zakrzewski, V. G.; Voth, G. A.; Salvador, P.; Dannenberg, J. J.; Dapprich, S.; Daniels, A. D.; Farkas, O.; Foresman, J. B.; Ortiz, J. V.; Cioslowski, J.; Fox,D. J. Gaussian 09, Revision D.01, Gaussian, Inc., Wallingford, CT, 2013.
[33] Reed, A. E.; Curtiss, L. A.; Weinhold, F. Chem. Rev. 1988, 88, 899.
[34] Reed, A. E.; Weinstock, R. B.; Weinhold, F. J. Chem. Phys. 1985, 83, 735.
[35] Carpenter, J. E.; Weinhold, F. J. Mol. Struct. 1988, 169, 41.
[36] Zhao, D.; Zhang, J.; Xie, Z. Angew. Chem., Int. Ed. 2014, 53, 12902.
[37] Qiu, Z.; Xie, Z. Dalton Trans. 2014, 43, 4925.
[38] Wang, S. R.; Xie, Z. Organometallics 2012, 31, 4544.
[39] Qiu, Z.; Ren, S.; Xie, Z. Acc. Chem. Res. 2011, 44, 299.
[40] Dang, Y.; Qu, S.; Tao, Y.; Song, C.; Wang, Z. X. J. Org. Chem. 2014, 79, 9046.
[41] Dub, P. A.; Béthegnies, A.; Poli, R. Organometallics 2012, 31, 294.
[42] Godbout, N.; Salahub, D. R.; Andzelm, J.; Wimmer, E. Can. J. Chem. 1992, 70, 560.
[43] Sosa, C.; Andzelm, J.; Elkin, B. C.; Wimmer, E.; Dobbs, K. D.; Dixon, D. A. J. Phys. Chem. 1992, 96, 6630.
[44] Miertus, S.; Scrocco, E.; Tomasi, J. Chem. Phys. 1981, 55, 117.
[45] Tao, J.-Y.; Mu, W.-H.; Chass, G. A.; Tang, T.-H.; Fang, D.-C. Int. J. Quantum Chem. 2013, 113, 975.
[46] Fang, D.-C. THERMO, Beijing Normal University, Beijing, China.
[47] Gonzalez, C.; Schlegel, H. B. J. Chem. Phys. 1989, 90, 2154.
[48] Gonzalez, C.; Schlegel, H. B. J. Phys. Chem. 1990, 94, 5523.
[49] Fukui, K. Acc. Chem. Res. 1981, 14, 363.
[50] Grimme, S. J. Comput. Chem. 2006, 27, 1787.
[51] Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. J. Chem. Phys. 2010, 132, 154104.
[52] Chai, J.-D.; Head-Gordon, M. Phys. Chem. Chem. Phys. 2008, 10, 6615.
[53] Zhao, Y.; Truhlar, D. G. J. Phys. Chem. 2006, 110, 5121.
[54] Mu, W. H.; Xia, S. Y.; Li, J. X.; Fang, D. C.; Wei, G.; Chass, G. A. J. Org. Chem. 2015, 80, 9108.
/
| 〈 |
|
〉 |