

Recent Advance in Asymmetric Trifluoromethylthiolation
Received date: 2018-07-30
Online published: 2018-08-29
Supported by
Project supported by the National Natural Science Foundation of China (No. 21332002) and the National Basic Research Program of China (973 Program, No. 2015CB856600).
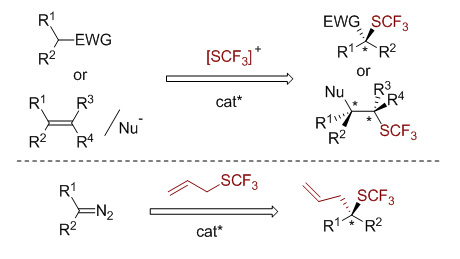
Fluorine-containing groups can modulate the physicochemical and biological properties of organic molecules. Consequently, the synthesis of fluorinated organic molecules has attracted considerable attention in the field of pharmaceuticals, agrochemicals and material sciences. Among fluorine-containing groups, the trifluoromethylthio group has the highest Hansch’s hydrophobicity parameter and remarkable electron-withdrawing character. The incorporation of a trifluoromethylthio group into organic molecules can significantly enhance their membrane permeability and metabolic stability because of its high lipophilicity and strong electron-withdrawing effect. As a result, various methods have been involved to synthesize SCF3-containing compounds using electrophilic or nucleophilic trifluoromethylthio reagents. On the other hand, the chirality of pharmaceutical molecules has an important effect on their properties, and different stereoisomers of a pharmaceutical molecules always have dramatically different pharmaceutical activities. Thus, the asymmetric trifluoromethylthiolation of organic molecules is of growing interest in recent years. Up to now, this field is still in the stage of initial development. In this perspective article, we will briefly summarize the methods of asymmetric trifluoromethylthiolation of organic molecules that have been reported so far. Two different strategies including the use of electrophilic trifluoromethylthiolating reagents and the use of trifluoromethylthio-containing building blocks will be introduced. Employing electrophilic trifluoromethylthiolating reagents, the enantioselective trifluoromethylthiolation of β-ketoesters, oxindoles as well as alkenes have been developed using Cinchona alkaloid, copper(Ⅱ) or indane-based chiral sulfide/selenide as the catalyst. Alternatively, using trifluoromethylthiolated building blocks is another approach to establish chiral centers bearing the trifluoromethylthio group. In this approach, an asymmetric trifluoromethylthiolation via enantioselective [2,3]-sigmatropic rearrangement of a sulfonium ylide generated from SCF3-containing sulfide and metal carbene has been disclosed using chiral Rh(Ⅱ) and Cu(I) as the catalyst. Finally, we will discuss the challenges of the asymmetric trifluoromethylthiolation of organic molecules in the future.
Li Shu-Sen , Wang Jianbo . Recent Advance in Asymmetric Trifluoromethylthiolation[J]. Acta Chimica Sinica, 2018 , 76(12) : 913 -924 . DOI: 10.6023/A18070306
[1] (a) Hagmann, W. K. J. Med. Chem. 2008, 51, 4359.
(b) Purser, S.; Moore, P. R.; Swallow, S.; Gouverneur, V. Chem. Soc. Rev. 2008, 37, 320.
(c) Manteau, B.; Pazenok, S.; Vors, J.-P.; Leroux, F. R. J. Fluorine Chem. 2010, 131, 140.
(d) Gillis, E. P.; Eastman, K. J.; Hill, M. D.; Donnelly, D. J.; Meanwell, N. A. J. Med. Chem. 2015, 58, 8315.
[2] (a) Leo, A.; Hansch, C.; Elkins, D. Chem. Rev. 1971, 71, 525.
(b) Hansch, C.; Leo, A.; Unger, S. H.; Kim, K. H.; Nikaitani, D.; Lien, E. J. J. Med. Chem. 1973, 16, 1207.
[3] For recent reviews on trifluoromethylthiolation, see:(a) Toulgoat, F.; Alazet, S.; Billard, T. Eur. J. Org. Chem. 2014, 2014, 2415.
(b) Shao, X.; Xu, C.; Lu, L.; Shen, Q. Acc. Chem. Res. 2015, 48, 1227.
(c) Xu, X.-H.; Matsuzaki, K.; Shibata, N. Chem. Rev. 2015, 115, 731.
(d) Zhang, K.; Xu, X.; Qing, F. Chin. J. Org. Chem. 2015, 35, 556(in Chinese). (张柯, 徐修华, 卿凤翎, 有机化学, 2015, 35, 556.)
(e) Xu, J.; Chen, P.; Ye, J.; Liu, G. Acta Chim. Sinica 2015, 73, 1294(in Chinese). (徐佳斌, 陈品红, 叶金星, 刘国生, 化学学报, 2015, 73, 1294.)
(f) Zheng, H.; Huang, Y.; Weng, Z. Tetrahedron Lett. 2016, 57, 1397.
(g) Li, G.; Sun, D. Chin. J. Org. Chem. 2016, 36, 1715(in Chinese). (李恭铭, 孙德群, 有机化学, 2016, 36, 1715).
(h) Barata-Vallejo, S.; Bonesi, S.; Postigo, A. Org. Biomol. Chem. 2016, 14, 7150.
(i) Guo, Y.; Huang, M.-W.; Fu, X.-L.; Liu, C.; Chen, Q.-Y.; Zhao, Z.-G.; Zeng, B.-Z.; Chen, J. Chin. Chem. Lett. 2017, 28, 719.
(j) Zhang, P.; Lu, L.; Shen, Q. Acta Chim. Sinica 2017, 75, 744(in Chinese). (张盼盼, 吕龙, 沈其龙, 化学学报, 2017, 75, 744.)(k)Zhao, X.; Li, T.; Tian, M.; Su, Z.; Wei, A.; Lu, K. Chin. J. Org. Chem. 2018, 38, 677(in Chinese). (赵霞, 李天骄, 田苗苗, 苏志扬, 魏奥琪, 芦逵, 有机化学, 2018, 38, 677.)
[4] Bootwicha, T.; Liu, X.; Pluta, R.; Atodiresei, I.; Rueping, M. Angew. Chem., Int. Ed. 2013, 52, 12856.
[5] Wang, X.; Yang, T.; Cheng, X.; Shen, Q. Angew. Chem., Int. Ed. 2013, 52, 12860.
[6] Rueping, M.; Liu, X.; Bootwicha, T.; Pluta, R.; Merkens, C. Chem. Commun. 2014, 50, 2508.
[7] Zhu, X.-L.; Xu, J.-H.; Cheng, D.-J.; Zhao, L.-J.; Liu, X.-Y.; Tan, B. Org. Lett. 2014, 16, 2192.
[8] Yang, T.; Shen, Q.; Lu, L. Chin. J. Chem. 2014, 32, 678.
[9] Liao, K.; Zhou, F.; Yu, J.-S.; Gao, W.-M.; Zhou, J. Chem. Commun. 2015, 51, 16255.
[10] Zhao, B.-L.; Du, D.-M. Org. Lett. 2017, 19, 1036.
[11] Li, M.; Xue, X.-S.; Cheng, J.-P. ACS Catal. 2017, 7, 7977.
[12] Deng, Q.-H.; Rettenmeier, C.; Wadepohl, H.; Gade, L. H. Chem.-Eur. J. 2014, 20, 93.
[13] Jin, M. Y.; Li, J.; Huang, R.; Zhou, Y.; Chung, L. W.; Wang, J. Chem. Commun. 2018, 54, 4581.
[14] Zhang, H.; Leng, X.; Wan, X.; Shen, Q. Org. Chem. Front. 2017, 4, 1051.
[15] Chachignon, H.; Kondrashov, E. V.; Cahard, D. Adv. Synth. Catal. 2018, 360, 965.
[16] Liu, X.; An, R.; Zhang, X.; Luo, J.; Zhao, X. Angew. Chem., Int. Ed. 2016, 55, 5846.
[17] Luo, J.; Liu, Y.; Zhao, X. Org. Lett. 2017, 19, 3434.
[18] Luo, J.; Cao, Q.; Cao, X.; Zhao, X. Nat. Commun. 2018, 9, 527.
[19] Liu, X.; Liang, Y.; Ji, J.; Luo, J.; Zhao, X. J. Am. Chem. Soc. 2018, 140, 4782.
[20] Zhang, Z.; Sheng, Z.; Yu, W.; Wu, G.; Zhang, R.; Chu, W.-D.; Zhang, Y.; Wang, J. Nat. Chem. 2017, 9, 970.
[21] Xu, L.; Wang, H.; Zheng, C.; Zhao, G. Adv. Synth. Catal. 2017, 359, 2942.
[22] Hock, K. J.; Koenigs, R. M. Angew. Chem., Int. Ed. 2017, 56, 13566.
[23] Mao, G.; Chen, L.; Wang, C. Sci. China Chem. 2017, 60, 1565.
/
| 〈 |
|
〉 |