

Wettability Analysis and Design of Micro-nanostructured Superhydrophobic Surface
Received date: 2018-10-16
Online published: 2018-12-03
Supported by
Project supported by the National Natural Science Foundation of China (Grant Nos. 11672024, 11372026) and National Basic Research Program of China (Grants No. 2015CB755803).
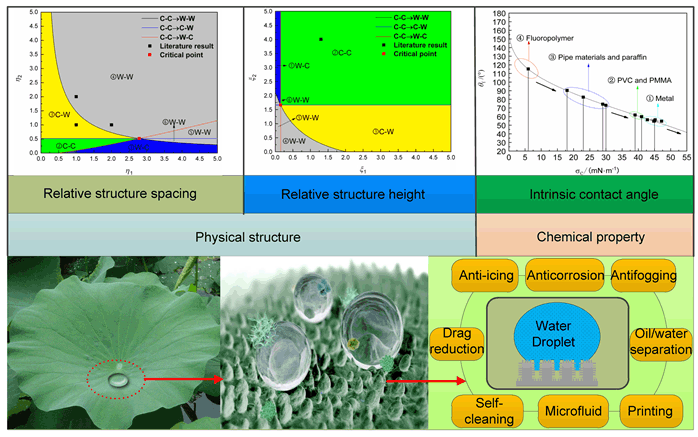
The special wettability of superhydrophobic surface usually has high contact angles (CA>150°) and low contact angle hysteresis (CAH<5°), which has been exploited for many potential applications. It is well known that wettability is mainly determined by micro/nano structure and surface composition, and various types of natural superhydrophobic surface could exhibit different wetting states, showing different wetting properties, such as low adhesive lotus leaf; the anisotropic superhydrophobic rice leaf; high adhesive rose petal. Therefore, the relationship between the wetting state and the surface structure should have a deeper understanding, especially in the design preliminary stage. The "droplet-superhydrophobic surface" system is taken as the research objects, four stable wetting state expressions are analyzed based on the principle of minimum energy. Wetting state transitions are studied on superhydrophobic surface coverd with micro/nano structured pillars of different distributions. The calculation formula of intrinsic contact angle is derived and the intrinsic contact angle of common materials is investigated. Based on the four wetting state of apparent contact angle equations, wetting diagrams were drew for investigating the wetting behavior, which include "one point, three lines, six areas, four state". The influence of the relative structure spacing and relative structure height on the wetting state is analyzed. It is found that the larger relative structure height, the smaller relative structure spacing, which can reduce the critical parameters of the transition state of the infiltration state, thereby expanding the range of the superhydrophobic surface, the more design options are available. It is also beneficial to the stability of the superhydrophobic surface, but should be controlled within certain scales because of mechanical stability. The simulation results accurately reflect the wetting state with the changes of the relative structure spacing and relative structure height. Finally, the general design of the superhydrophobic surface is refined. The results can provide theoretical guidance and technical fundament for the design of superhydrophobic surfaces.
Ma Guojia , Zheng Haikun , Chang Shinan , Wang Shuoshuo . Wettability Analysis and Design of Micro-nanostructured Superhydrophobic Surface[J]. Acta Chimica Sinica, 2019 , 77(3) : 269 -277 . DOI: 10.6023/A18100430
[1] Barthlott, W.; Neinhuis, C. Planta 1997, 202, 1.
[2] Marmur, A. Langmuir 2003, 19, 5956.
[3] Zheng, H. K.; Chang, S. N.; Zhao, Y. Y. Prog. Chem. 2017, 29, 102. (郑海坤, 常士楠, 赵媛媛, 化学进展, 2017, 29, 102.)
[4] Kreder, M. J.; Alvarenga, J.; Kim, P.; Aizenberg, J. Nat. Rev. Mater. 2016, 1, 15003.
[5] Liang, W. X.; Zhang, Y. B.; Wang, B.; Guo, Z. G.; Liu, W. M. Acta Chim. Sinica 2012, 70, 2393(梁伟欣, 张亚斌, 王奔, 郭志光, 刘维民, 化学学报, 2012, 70, 2393.)
[6] Zhang, J. N.; Yu, J. H. Chin. J. Chem. 2018, 36, 51.
[7] Cui, L. Y.; Fan, S. S.; Yu, C. L.; Kuang, M. X.; Wang, J. X. Acta Chim. Sinica 2017, 75, 967. (崔丽影, 范莎莎, 于存龙, 邝旻翾, 王京霞, 化学学报, 2017, 75, 967.)
[8] Wenzel, R. N. Ind. Eng. Chem. 1936, 28, 988.
[9] Cassie, A. B. D.; Baxter, S. Trans. Faraday Soc. 1944, 40, 546.
[10] Bico, J.; Tiele, U.; Quere, D. Colloid Surface A 2002, 206, 41.
[11] Bhushan, B.; Nosonovsky, M. Phil. Trans. R. Soc. A 2010, 368, 4713.
[12] Suzuki, S.; Ueno, K. Langmuir 2017, 33, 138.
[13] Hejazi, V.; Nosonovsky, M. Colloid Polym. Sci. 2013, 291, 329.
[14] Tuvshindorj, U.; Yildirim, A.; Ozturk, F. E.; Bayindir, M. ACS Appl. Mater. Inter. 2014, 6, 9680.
[15] Wang, S.; Jiang, L. Adv. Mater. 2007, 19, 3423.
[16] Rahmawan, Y.; Moon, M. W.; Kim, K. S. Langmuir 2009, 26, 484.
[17] Wu, B. B.; Wu, H. P.; Zhang, Z.; Dong, C. C.; Chai, G. Z. Acta Phys. Sin. 2015, 64, 176801. (吴兵兵, 吴化平, 张征, 董晨晨, 柴国钟, 物理学报, 2015, 64, 176801.)
[18] Lv, P. Y.; Xue, Y. H.; Duan, H. L. Adv. Mech. 2016, 46, 179. (吕鹏宇, 薛亚辉, 段慧玲, 力学进展, 2016, 46, 179.)
[19] Moosmann, M.; Schimmel, T.; Barthlott, W.; Mail, M. Beilstein J. Nanotech. 2017, 8, 1671.
[20] Seo, J.; García-Mayoral, R.; Mani, A. J. Fluid Mech. 2018, 8, 35.
[21] Lafuma, A.; Queré, D. Nat. Mater. 2003, 2, 457.
[22] Ye, X. M.; Zhang, X. S.; Li, M. L.; Li, C. X. Acta Phys. Sin. 2018, 67, 114702. (叶学民, 张湘珊, 李明兰, 李春曦, 物理学报, 2018, 67, 114702.)
[23] Kwon, H. M.; Paxson, A. T.; Varanasi, K. K.; Patankar, N. A. Phys. Rev. Lett. 2011, 106, 036102.
[24] Bartolo, D.; Bouamrirene, F.; Verneuil, É.; Buguin, A.; Silberzan, P.; Moulinet, S. EPL 2006, 74, 299.
[25] Bormashenko, E.; Pogreb, R.; Whyman, G.; Erlich, G. Langmuir 2007, 23, 12217.
[26] Girifalco, L. A.; Good, R. J. J. Phys. Chem. 1957, 61, 904.
[27] Zisman, W. A. Adv. Chem. 1964, 43, 1.
[28] Barthlott, W.; Neinhuis, C.; Cutler, D.; Ditsch, F.; Meusel, I.; Theisen, I. Bot. J. Linn. Soc. 1998, 126, 237.
[29] Nishino, T.; Meguro, M.; Nakamae, K. Langmuir 1999, 15, 4321.
[30] Han, J. T.; Sun, Y. K.; Woo, J. S.; Lee, G. W. Adv. Mater. 2010, 20, 3724.
[31] Raza, M. A.; Kooij, E. S.; Silfhout, A. Langmuir 2010, 26, 12962.
[32] Liu, X.; Dai, B.; Zhou, L.; Sun, J. J. Mater. Chem. 2009, 19, 497.
[33] Dong, J.; Jin, Y.; Dong, H.; Sun, L. Langmuir 2017, 33, 1041.
[34] Guan, Z. S.; Zhang, Q. Acta Chim. Sinica 2005, 63, 880. (管自生, 张强, 化学学报, 2005, 63, 880.)
[35] Kulinich, S. A.; Farhadi, S.; Nose, K. Langmuir 2011, 27, 25.
/
| 〈 |
|
〉 |