

A Ratiometric Fluorescence Probe for Detecting Gaseous Isocyanates Directly
Received date: 2018-12-04
Online published: 2019-01-31
Supported by
Project supported by the Natural Science Foundation of Guangdong Province, China (No. 2018A030310348), and the Guangzhou Science and Technology Plan (No. 201803030031).
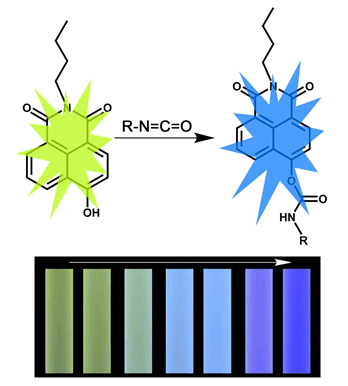
Isocyanates is a widely-used chemical in many manufacturing industries, such as polymer industry, pharmaceutical production and production of a variety of agricultural chemicals. However, it is harmful to human health due to the volatility. Therefore, it is necessary to develop methods to detect isocyanates quickly and conveniently, especially to gaseous isocyanates. In this work, a novel fluorescent probe, N-buty-4-hydroxy-1,8naphthalimide, was developed for detection of isocyanates. This fluorescence probe can be synthesized by a simple three-steps synthetic route, and the overall yield of the whole synthesizing process reached 75%. In the absence of isocyanate, the probe solution displays an emission centering at 596 nm when excited at 370 nm, which is yellow to the naked eye. Once isocyanate is added, the fluorescence of solution changes from yellow to blue, and the process finishes in 4 min. The detecting limit of this probe to isocyanates is calculated to be 112 nmol·L-1. It is also proved that this probe possesses excellent selectivity for isocyanate and distinct anti-interference to common organic volatilized compounds. In addition, the reaction mechanism between the probe and isocyanate were proved by HPLC, NMR and ESI-MASS. Results show that the hydroxy group on the 4th position of naphthalene ring of probe reacts with isocyanate group (-NCO) of isocyanate, and resulting in carbamates, which alter 4th substituent group of probe molecule and lead to change of fluorescence. In order to detect the gaseous isocyanates directly, test paper are developed based on N-buty-4-hydroxy-1,8naphthalimide. When the test paper exposed to isocyanates vapor, the yellow fluorescence fade away gradually and a blue fluorescence appear in 6 min. And the test paper possesses excellent selectivity for gaseous isocyanate and distinct anti-interference to common VOCs. In conclusion, this strategy is an efficient way to detect gaseous isocyanates, and it may provide a referable approach for directly monitoring the volatile organic compounds in air.
Key words: isocyanates; fluorescence probe; testing paper
Chen Kai , Han Baichuan , Ji Sixin , Sun Jin , Gao Zhenzhong , Hou Xianfeng . A Ratiometric Fluorescence Probe for Detecting Gaseous Isocyanates Directly[J]. Acta Chimica Sinica, 2019 , 77(4) : 365 -370 . DOI: 10.6023/A18120484
[1] Xuan, L. M. S. Thesis, North China University of Science and Technology, Tangshan, 2015(in Chinese). (宣靓, 硕士论文, 华北理工大学, 唐山, 2015.)
[2] Tang, Y. F. M. S. Thesis, Anhui Jianzhu University, Hefei, 2013(in Chinese). (唐燕飞, 硕士论文, 安徽建筑大学, 合肥, 2013.)
[3] Spindler, R.; Frechet, J. M. J. Macromolecules 1993, 26, 4809.
[4] Xie, W.-Q.; Chai, X.-S. J. Chromatogr. A 2016, 1468, 241.
[5] Kubitz, K. A. Anal. Chem. 1957, 29, 814.
[6] Kormos, L. H.; Sandridge, R. L.; Keller, J. Anal. Chem. 1981, 53, 1122.
[7] Budnik, L. T.; Nowak, D.; Merget, R.; Lemiere, C.; Baur, X. J. Occup. Med. Toxicol. 2011, 6, 9.
[8] Coldwell, M.; White, J. UK Health and Safety Laboratory, Buxton, 2005.
[9] Agarwal, B.; Jurschik, S.; Sulzer, P. Rapid Commun. Mass Spectrom. 2012, 26, 983.
[10] Andre, C.; Jorge, F.; Castanheira, I.; Matos, A. J. Chemometr. 2013, 27, 91.
[11] Karlsson, D. Anal. Chim. Acta 2005, 534, 263.
[12] NIOSH Pocket Guide to Chemical Hazards and Other Databases. DHHS(NIOSH) Publication No. 2001-145(2001).
[13] Skarping, G.; Renman, L.; Smith, B. E. F. J. Chromatogr. A 1983, 267, 315.
[14] Sharping, G.; Sango, C.; Smith, B. E. F. J. Chromatogr. A 1981, 208, 313.
[15] Dunlap, K. L.; Sandridge, R. L.; Keller, J. Anal. Chem. 1976, 48, 497.
[16] Gagne, S.; Lesage, J.; Ostiguy, C.; Van Tra, H. Analyst 2003, 128, 1447.
[17] Warwick, C. J.; Bagon, D. A.; Purnell, C. J. Analyst 1981, 106, 676.
[18] Ghosh, K. R.; Saha, S. K.; Gao, J.-P.; Wang, Z.-Y. Chem. Commun. 2014, 50, 1886.
[19] Gao, Z.-Z.; Han, B.-C.; Chen, K. Chem. Commun. 2017, 53, 6231.
[20] Chen, Q.; Bian, N.; Cao, C.; Qiu, X.-L.; Qi, A.-D.; Han, B.-H. Chem. Commun. 2010, 46, 4067.
[21] Li, Q.-Q.; Li, Z. Adv. Sci. 2017, 4, 1600484.
[22] Chen, J.; Zhang, C.-H.; Lv, K.; Wang, H.; Zhang, P.-S.; Yi, P.-G.; Jiang, J.-H. Sensor. Actuat. B 2017, 251, 533.
[23] Daniela, G.; Alicia, V. V.; Denis, B.; Angel, G. B. J. Nanophotonics 2018, 12, 012505.
[24] Zhang, G.-X.; Hu, F.; Zhang, D.-Q. Langmuir 2015, 31, 4593.
[25] Sun, C.-L.; Teng, K.-X.; Niu, L.-Y.; Chen, Y.-Z.; Yang, Q.-Z. Acta Chim. Sinica 2018, 76, 779(in Chinese). (孙才力, 滕坤旭, 牛丽亚, 陈玉哲, 杨清正, 化学学报, 2018, 76, 779.)
[26] Wang, J.; Wu, Y.-L.; Sun, L.-H.; Zeng, F.; Wu, S.-Z. Acta Chim. Sinica 2016, 74, 910(in Chinese). (王俊, 武英龙, 孙立和, 曾钫, 吴水珠, 化学学报, 2016, 74, 910.)
[27] Liu, Z.; He, W.; Guo, Z. Chem. Soc. Rev. 2013, 42, 1568.
[28] Valeur, B.; Leray, I. Coord. Chem. Rev. 2000, 20, 3.
[29] Zheng, X.-J.; Zhu, W.-C.; Liu, D.; Ai, H. Appl. Mater. Interfaces 2014, 6, 7996.
[30] Lin, H.-H.; Chan, Y.-C.; Chen, J.-W. J. Mater. Chem. 2011, 21, 3170.
[31] Leng, B.; Jiang, J.-B.; Tian, H. AlChE J. 2010, 56, 2957.
[32] Kai, Y.-M.; Hu, Y.-H.; Wang, K.; Zhi, W.-B.; Liang, M.-M.; Yang, M. Spectrochim. Acta A 2014, 118, 239.
[33] Zhang, H.-F.; Liu, T.; Yin, C.-C.; Wen, Y.; Chao, J.-B.; Zhang, Y.-B.; Huo, F.-J. Spectrochim. Acta A 2017, 174, 230.
[34] Li, B.; Yu, Y.-L.; Xiang, F.-Q.; Zhang, S.-Y. Appl. Mater. Interfaces 2018, 10, 16282.
[35] Guo, B.-P.; Pan, X.-Z.; Liu, Y.-F.; Nie, L.-X.; Zhao, H.-Z.; Liu, Y.-Z.; Jing, J.; Zhang, X.-L. Sensor. Actuat. B-Chem. 2018, 256, 632.
[36] Zhang, Z.; Fan, J.-L.; Zhao, Y.-H.; Kang, Y.; Du, J.-J.; Peng, X.-J. ACS Sens. 2018, 3, 735.
/
| 〈 |
|
〉 |