

Intramolecular and Intermolecular Methyl Migration of Fenthion Studied by Electrospray Ionization Mass Spectrometry
Received date: 2018-12-17
Online published: 2019-02-25
Supported by
Project supported by the National Natural Science Foundation of China (No. 21427802), the National Natural Science Foundation of China (No. 21520102007, 21605017), Project of Jiangxi Provincial Department of Education (No. GJJ160574), and the Jiangxi Key Laboratory for Mass Spectrometry and Instrumentation Open Fund (JXMS201803).
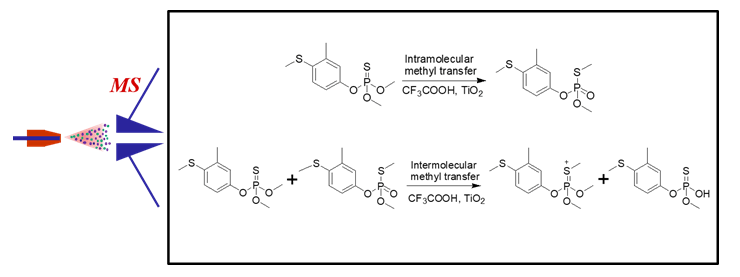
Methyl transfer reactions are of great significance in the field of synthetic chemistry and life sciences. So far, most of the reported methyl migration reactions have occurred between different types of molecules. Therefore, it is of certain value to search for new methyl transfer reactions. In this study, fenthion, a most common insecticide in the environment, was selected as the studied object, and electrospray ionization mass spectrometry (ESI-MS) was used as the analytical tool to conduct highly sensitive analysis of the reaction system, so as to explore the possibility of methyl transfer reaction in fenthion molecules under the condition of trifluoroacetic acid and nanometer titanium dioxide. Other than m/z 279 (protonated fenthion), some new product ions (m/z 293 and m/z 265) could be observed in the fingerprint MS of fenthion reaction solution. Tandem MS experiments showed that the intensity of product ion m/z 231 (elimination of CH3SH) in the dissociation of m/z 279 from fenthion reaction solution were different from that from protonated fenthion standard. This indicated that the methyl in the fenthion could transfer from oxygen atom to unsaturated sulfur atom via 1,3-methyl transfer, forming isomer a2, which led to the high intensity of product ion m/z 231 in the dissociation of m/z 279 from fenthion reaction solution. Under the assistance of acid, the methyl cation continued to transfer from sulfur atom in a2 to the unsaturated sulfur atom in another fenthion molecule, forming a3 (m/z 293) and a4 via intermolecular methyl transfer reaction, which was verified by tandem MS experiments of ions at m/z 293 and m/z 265. In addition, density functional theory (DFT) calculations were carried out to confirm the mechanism of intramolecular and intermolecular methyl transfer reactions of fenthion. In order to observe the phenomenon of methyl transfer more intuitively, the effects of different acids, metal oxides, reaction time and reaction temperature on the signal intensities of ions at m/z 265 and m/z 293 of intermolecular methyl transfer reactions of fenthion were investigated. It could be concluded that under the conditions of trifluoroacetic acid and nanometer titanium dioxide, and 60℃ ultrasound reaction for 6 h, the proportion of intermolecular methyl transfer reactions of fenthion was the highest. In this study, intramolecular and intermolecular methyl transfer reactions were both discovered and investigated in fenthion, which can not only provide a method to study methyl transfer reactions, but also propose a new idea for the study of degradation of fenthion.
Ren Xiang , Zhang Xiaoping , Wang Yufen , Cao Jingyu , Cheng Yuanyuan , Feng Shouhua , Chen Huanwen . Intramolecular and Intermolecular Methyl Migration of Fenthion Studied by Electrospray Ionization Mass Spectrometry[J]. Acta Chimica Sinica, 2019 , 77(4) : 358 -364 . DOI: 10.6023/A18120505
[1] Dakternieks, D.; Lim, A. E. K.; Lim, K. F. Chem. Commun. 1999, 15, 1425.
[2] Shekar, S.; Brown, S. N. J. Org. Chem. 2014, 79, 12047.
[3] Schmidt, T.; Schwede, T.; Meuwly, M. J. Phys. Chem. B 2014, 118, 5882.
[4] Lukinavicius, G.; Lapiene, V.; Stasevskij, Z.; Dalhoff, C.; Weinhold, E.; Klimasauskas, S. J. Am. Chem. Soc. 2007, 129, 2758.
[5] Khaskin, E.; Zavalij, P. Y.; Vedernikov, A. N. J. Am. Chem. Soc. 2008, 130, 10088.
[6] Shekar, S.; Brown, S. N. Organometallics 2013, 32, 556.
[7] Jones, P. A.; Takai, D. Science 2001, 293, 1068.
[8] Klose, R. J.; Bird, A. P. Trends Biochem. Sci. 2006, 31, 89.
[9] Finnegan, E. J.; Peacock, W. J.; Dennis, E. S. Curr. Opin. Genet. Dev. 2000, 10, 217.
[10] Xiong, L.; Ping, L.; Yuan, B.; Wang, Y. J. Am. Soc. Mass Spectrom. 2009, 20, 1172.
[11] Hughes, R. M.; Waters, M. L. J. Am. Chem. Soc. 2005, 127, 6518.
[12] Callahan, B. P.; Wolfenden, R. J. Am. Chem. Soc. 2003, 125, 310.
[13] Zhang, X.; Wang, H.; Liao, Y.; Ji, H.; Guo, Y. J. Mass Spectrom. 2010, 42, 218.
[14] Kshirsagar, U. A.; Argade, N. P. Tetrahedron 2009, 65, 5244.
[15] Zhang, X. J. Mol. Struc.-Theochem. 2010, 955, 91.
[16] Zhang, X.; Yao, S.; Guo, Y. Int. J. Mass Spectrom. 2008, 270, 31.
[17] Reepmeyer, J. C. Rapid Commun. Mass Spectrom. 2010, 23, 927.
[18] Gao, X.; Zhu, G.; Zeng, Z.; Chen, W.; Lin, Z.; Liu, Y.; Xu, P.; Zhao, Y. Rapid Commun. Mass Spectrom. 2011, 25, 1061.
[19] Ammal, S. C.; Yamataka, H.; Aida, M. A.; Dupuis, M. Science 2003, 299, 1555.
[20] Kitamura, S.; Kadota, T.; Yoshida, M.; Jinno, N.; Ohta, S. Comp. Biochem. Phys. C 2000, 126, 259.
[21] Bai, C. L.; Qiao, C. B.; Zhang, W. D.; Chen, Y. L.; Qu, S. X. Biomed. Environ. Sci. 1990, 3, 262.
[22] Ben Amara, I.; Sefi, M.; Troudi, A.; Soudani, N.; Boudawara, T.; Zeghal, N. Indian J. Biochem. Bio. 2014, 51, 293.
[23] Cheke, R. A.; Mcwilliam, A. N.; Mbereki, C.; Van Der Walt, E.; Mtobesya, B.; Magoma, R. N.; Young, S.; Eberly, J. P. Ecotoxicology 2012, 21, 1761.
[24] Celik, I.; Isik, I.; Ozok, N.; Kaya, M. S. Toxicol. Ind. Health 2011, 27, 357.
[25] Eckstein, F.; Gish, G. Trends Biochem. Sci. 1989, 14, 97.
[26] Frey, P. A.; Sammons, R. D. Science 1985, 228, 541.
[27] Wilkins, E.; Carter, M.; Voss, J.; Ivnitski, D. Electrochem. Commun. 2000, 2, 786.
[28] Chen, P. S.; Huang, S. D. Talanta 2006, 69, 669.
[29] Salm, P.; Taylor, P. J.; Roberts, D.; Silva, J. D. J. Chromatogr. B 2009, 877, 568.
[30] You, Z.-S.; Wen, Y.-J.; Jiang, K.-Z.; Pan, Y.-J. Chin. Sci. Bull. 2012, 57, 1183. (尤珠双, 文永均, 蒋可志, 潘远江, 科学通报, 2012, 57, 1183.)
[31] Zhang, J.; Chai, Y. F.; Wang, W.; Shang, W.; Pan, Y. J. Chinese J. Chem. 2012, 30, 2383.
[32] Chai, Y.-F.; Gan, S.-F.; Pan, Y.-J. Acta Chim. Sinica 2012, 70, 1805. (柴云峰, 甘世凤, 潘远江, 化学学报, 2012, 70, 1805.)
[33] Yin, X.-C.; Jiang, Y.; Chu, S.-Y.; Weng, G.-F.; Fang, X.; Pan, Y.-J. Acta Chim. Sinica 2018, 76, 436. (尹欣驰, 江游, 楚士颖, 翁国锋, 方向, 潘远江, 化学学报, 2018, 76, 436.)
[34] Zhang, X. P.; Chen, H. H.; Ji, Y.; Jiang, K. Z.; Chen, H. W. J. Am. Soc. Mass Spectrom. 2018, doi.org/10.1007/s13361-018-2098-4.
[35] Newman, M. S.; Karnes, H. A. J. Org. Chem. 1966, 31, 3980.
/
| 〈 |
|
〉 |