

Molecular Dynamics Simulation of the Stability Behavior of Graphene in Glycerol/Urea Solvents in Liquid-Phase Exfoliation
Received date: 2020-10-13
Online published: 2021-02-22
Supported by
National Natural Science Foundation of China(51603160)
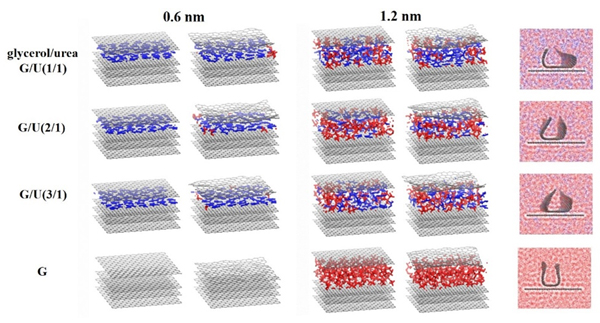
Understanding of interfacial structure and stabilization mechanism of graphene sheets in green solvents during liquid-phase exfoliation is of great importance in advancing preparation, characterization and synthesis of graphene-based materials. However, it is difficult to monitor structural evolution of graphene in solvents using current available experimental techniques, and the resulting graphene stabilization mechanism has not been fully understood. In this work, molecular dynamics simulations are performed to investigate the structural evolution and stability behavior of the graphene sheets with different states in the glycerol/urea green solvents with varying concentrations. The results show that only the pristine graphene sheet at an initial interlayer spacing of 0.6 nm in the glycerol solvent restacks back and stays close to each other at a separation close to the intrinsic thickness of graphene. This is due to the fact that the glycerol molecules fail to diffuse into the graphene interlayer, and they could not afford a sufficient repulsive barrier to hinder the graphene aggregation. While in other pristine cases, a single- or double-layer solvent structure is formed and the interlayer separation is maintained at 0.65 nm or 1.12~1.18 nm, offering an efficient dispersion medium to stabilize the graphene sheets. Although the pristine multilayer graphene sheets present similar stability in different glycerol/urea solvents, the U-type graphene experiences distinct levels of stabilization in solvents in the order of pure glycerol>glycerol/urea(2/1)>glycerol/urea(3/1)>glycerol/urea(1/1), signifying that the shifted and exfoliated state of the graphene sheets plays an important role in the stabilization during liquid-phase exfoliation. Moreover, in the glycerol/urea binary solvents, the small urea molecules firstly diffuse into the graphene interlayer due to their strong π-π interaction with graphene, acting as a “dispersion initiator”. And then the glycerol molecules could have the chance to diffuse around or insert into the graphene interlayer to assist in stabilizing the graphene due to the hydrogen bonding between urea and glycerol. In this way, the glycerol helps to further increase the interlayer separation leading to a more stable dispersion, acting as a “dispersion co-stabilizer”. The formation of the confined urea and glycerol solvents thus provides a stable molecular layer structure between the graphene interlayer, enabling to stabilize the exfoliated graphene sheets effectively. As such, the findings in this work are believed to provide the atomic/molecular scale understanding of the stability behavior of the graphene sheets in glycerol/urea binary solvents during liquid-phase exfoliation.
Key words: molecular dynamics; stability; graphene; glycerol; urea
Chang-An Liu , Shi-Bo Hong , Bei Li . Molecular Dynamics Simulation of the Stability Behavior of Graphene in Glycerol/Urea Solvents in Liquid-Phase Exfoliation[J]. Acta Chimica Sinica, 2021 , 79(4) : 530 -538 . DOI: 10.6023/A20100468
| [1] | Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Science 2004, 306,666. |
| [2] | Hu, Y.J.; Jin, J.; Zhang, F.; Wu, P.; Cai, C.X. Acta Phys.-Chim. Sinica 2010, 26,2073 . . (in Chinese) |
| [2] | ( 胡耀娟, 金娟, 张卉, 吴萍, 蔡称心, 物理化学学报, 2010, 26,2073.) |
| [3] | Fan, S.Q.; Tan, R.X.; Xie, X.M.; Zhang, M.Y.; Huang, Q.Z. New Carbon Mater. 2018, 33,522. (in Chinese) |
| [3] | ( 樊姝婧, 谭瑞轩, 谢翔旻, 张明瑜, 黄启忠, 新型炭材料, 2018, 33,522.) |
| [4] | Le, M.Q. Int. J. Mech. Mater. Des. 2015, 11,15. |
| [5] | Li, X.Y.; Wang, J.; Wu, R. J. Chin. J. At.Mol. Phys. 2018, 35,139. (in Chinese) |
| [5] | ( 李旭艳, 王静, 吴荣, 原子与分子物理学报, 2018, 35,139.) |
| [6] | Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Science 2008, 321,385. |
| [7] | Chen, J.H.; Jang, C.; Xiao, S.; Ishigami, M.; Fuhrer, M.S. Nat. Nanotechnol. 2008, 3,206. |
| [8] | Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Nano Lett. 2008, 8,902. |
| [9] | Liu, X. Ph.D. Dissertation, Donghua University, Shanghai, 2016. (in Chinese) |
| [9] | ( 刘霞 , 博士论文, 东华大学, 上海, 2016.) |
| [10] | Walker, L.S.; Marotto, V.R.; Rafiee, M.A.; Koratkar, N.; Corral, E.L. ACS Nano 2011, 5,3182. |
| [11] | Kim, H.; Abdala, A.A.; Macosko, C.W. Macromolecules 2010, 43,6515. |
| [12] | Bai, H.; Li, C.; Shi, G. Adv. Mater. 2011, 23,1089. |
| [13] | Geng, Z.; Hähnlein, B.; Granzner, R.; Auge, M.; Ledeveb, A.A.; Davydov, V.Y.; Kitller, M.; Pezoldt, J.; Schwierz, F. Ann. Phys. 2017, 529,1700033. |
| [14] | Wang, H.; Kurata, K.; Fukunaga, T.; Ago, H.; Takamatsu, H.; Zhang, X.; Ikuta, T.; Takahashi, K.; Nishiyama, T.; Takata, Y. Sens. Actuators, A 2016, 247,24. |
| [15] | Ma, C. M.S. Thesis, Harbin Institute of Technology, Harbin, 2015. (in Chinese) |
| [15] | ( 马聪 , 硕士论文, 哈尔滨工业大学, 哈尔滨, 2015.) |
| [16] | Tan, Y.B.; Lee, J.M. J. Mater. Chem. A 2013, 1,14814. |
| [17] | Liu, Y.; Tao, W.; Wu, D. Chin. J. Chem. 2020, 38,1123. |
| [18] | Hill, E.W.; Vijayaragahvan, A.; Novoselov, K. IEEE Sens. J. 2011, 11,3161. |
| [19] | Mackin, C.; Schroeder, V.; Zurutuza, A.; Su, C.; Kong, J.; Swager, T.M.; Palacios, T. ACS Appl. Mater. Interfaces 2018, 10,16169. |
| [20] | Miao, J.; Shi, Y.; Zhu, H.; Gao, M. Chin. J. Chem. 2020, 38,719. |
| [21] | Zhang, H.; Gruener, G.; Zhao, Y. J. Mater. Chem. B 2013, 1,2542. |
| [22] | Byun, J.J. Microbiol. Biotechnol. 2015, 25,145. |
| [23] | Lu, J.; Tan, S.; Zhu, Y.; Li, W.; Chen, T.; Wang, Y.; Liu, C. Acta Chim. Sinica 2019, 77,253. (in Chinese) |
| [23] | ( 卢静荷, 谭淑珍, 朱雨清, 李伟, 陈天啸, 王瑶, 刘陈, 化学学报, 2019, 77,253.) |
| [24] | Song, G.; Wu, T.; Liu, F.; Zhang, B.; Liu, X. Acta Chim. Sinica 2020, 78,82. (in Chinese) |
| [24] | ( 宋光捷, 武调弟, 刘福鑫, 张彬雁, 刘秀辉, 化学学报, 2020, 78,82.) |
| [25] | Meyer, J.C.; Geim, A.K.; Katsnelson, M.I.; Novoselov, K.S.; Booth, T.J.; Roth, S. Nature 2007, 446,60. |
| [26] | Sutter, P.W.; Flege, J.I. Nat. Mater. 2008, 7,406. |
| [27] | Emtsev, K.V.; Bostwick, A.; Horn, K.; Jobst, J.; Kellogg, G.L.; Ley, L.; McChesney, J.L.; Ohta, T.; Reshanov, S.A.; Rohrl, J.; Rotenberg, E.; Schmid, A.K.; Waldmann, D.; Weber, H.B.; Seyller, T. Nat. Mater. 2009, 8,203. |
| [28] | Li, X.; Cai, W.; An, J.; Kim, S.; Nah, J.; Yang, D.; Piner, R.; Velamakanni, A.; Jung, I.; Tutuc, E.; Banerjee, S.K.; Colombo, L.; Ruoff, R.S. Science 2009, 324,1312. |
| [29] | Chae, S.J.; Güneş, F.; Kim, K.K.; Kim, E.S.; Han, G.E.; Kim, S.M.; Shin, H.; Yoon, S.; Choi, J.; Park, M.H.; Yang, C.W; Pribat, D.; Lee, Y.H. Adv. Mater. 2009, 21,2328. |
| [30] | Zhu, Y.; Stoller, M.D.; Cai, W.; Velamakanni, A.; Piner, R.D.; Chen., D.; Ruoff,, R.S . ACS Nano 2010, 4,1227. |
| [31] | Chen, W.; Yan, L. Nanoscale 2010, 2,559. |
| [32] | Hernandez, Y.; Nicolosi, V.; Lotya, M.; Blighe, F.M.; Sun, Z.; De, S.; McGovern, I.T.; Holland, B.; Byrne, M.; GunKo, Y.K.; Boland, J.J.; Niraj, P.; Duesberg, G.; Krishnamurthy, S.; Goodhue, R.; Hutchison, J.; Scardaci, V.; Ferrari, A.C.; Coleman, J.N. Nat. Nanotechnol. 2008, 3,563. |
| [33] | Hernandez, Y.; Lotya, M.; Rickard, D.; Bergin, S.D.; Coleman, J.N. Langmuir 2010, 26,3208. |
| [34] | Lotya, M.; Hernandez, Y.; King, P.J.; Smith, R.J.; Nicolosi, V.; Karlsson, L.S.; Blighe, F.M.; De, S.; Wang, Z.; McGovern, I.T.; Duesberg, G.S.; Coleman, J.N. J. Am. Chem. Soc. 2009, 131,3611. |
| [35] | Coleman, J.N. Adv. Funct. Mater. 2009, 19,3680. |
| [36] | Coleman, J.N. Acc. Chem. Res. 2013, 46,14. |
| [37] | Shih, C.J.; Lin, S.; Strano, M.S.; Blankschtein, D. J. Am. Chem. Soc. 2010, 132,14638. |
| [38] | Fu, C.; Yang, X. Carbon 2013, 55,350. |
| [39] | Yang, J.; Yang, X.; Li, Y. Curr. Opin. Colloid Interface Sci. 2015, 20,339. |
| [40] | Xu, X.; Cai, L.; Zheng, X.; Xu, Q. Phys. Chem. Chem. Phys. 2017, 19,16062. |
| [41] | Chiu, P.L.; Mastrogiovanni, D.D. T.; Wei, D.; Louis, C.; Jeong, M.; Yu, G.; Saad, P.; Flach, C.R.; Mendelsohn, R.; Garfunkel, E.; He, H. J. Am. Chem. Soc. 2012, 134,5850. |
| [42] | Shi, M.Y.; Zhang, X.F.; Wang, X.Y.; Wang, W.Z.; Jiang, X.Q. J. Nanjing Norm. Univ. (Engineering and Technology) 2014, 14,1. (in Chinese) |
| [42] | ( 石梦燕, 张晓凤, 王孝英, 王文珠, 蒋晓青, 南京师范大学学报: 工程技术版, 2014, 14,1.) |
| [43] | Chen, J.; Shi, W.; Gao, Z.; Wang, T.; Wang, S.; Dong, L.; Yang, Q.; Xiong, C. Nano Res. 2018, 11,820. |
| [44] | Kim, H.S.; Oweida, T.J.; Yingling, Y.G. J. Mater. Sci. 2018, 53,5766. |
| [45] | Kim, H.S.; Huang, S.M.; Yingling, Y.G. MRS Adv. 2016, 1,1883. |
| [46] | Kim, H.S.; Farmer, B.L.; Yingling, Y.G. Adv. Mater. Interfaces 2017, 4,1601168. |
| [47] | Sun, J.; Li, Y.; Lin, J. J. Mol. Graphics Modell. 2017, 74,16. |
| [48] | da Silva, A.W. S.; Vranken, W.F. BMC Res. Notes 2012, 5,367. |
| [49] | Mandell, M.J.; McTague, J.P.; Rahman, A. J. Chem. Phys. 1976, 64,3699. |
| [50] | Hess, B.; Bekker, H.; Berendsen, H.J. C.; Fraaije, J. G. E.M.. J. Comput. Chem. 1997, 18,1463. |
| [51] | Hess, B. J. Chem. Theory Comput. 2008, 4,116. |
| [52] | Darden, T.; York, D.; Pedersen, L. J. Chem. Phys. 1998, 98,10089. |
| [53] | Essmann, U.; Perera, L.; Berkowitz, M.L.; Darden, T.; Lee, H.; Pedersen, L.G. J. Chem. Phys. 1995, 103,8577. |
| [54] | Yau, A.W.; Pritchard, H.O. Can. J. Chem. 1977, 55,992. |
| [55] | Berendsen, H.J. C.; Postma, J.P. M.; van Gunsteren, W.F.; DiNola, A.; Haak, J.R. J. Chem. Phys. 1984, 81,3684. |
| [56] | Bussi, G.; Donadio, D.; Parrinello, M. J. Chem. Phys. 2007, 126,14101. |
| [57] | Parrinello, M.; Rahman, A. J. Appl. Phys. 1981, 52,7182. |
| [58] | Hoover, W.G. Phys. Rev. A 1985, 31,1695. |
| [59] | Jussila, H.; Yang, H.; Granqvist, N.; Sun, Z. Optica 2016, 3,151. |
| [60] | Wolfe, M.; Jonas, J. J. Chem. Phys. 1979, 71,3252. |
| [61] | Lide, D.R. CRC handbook of chemistry and physics: a ready-reference book of chemical and physical data, CRC Press, New York, 1995. |
| [62] | Carugo, O.; Pongor, S. Protein Sci. 2001, 10,1470. |
| [63] | Moghaddam, M.B.; Goharshadi, E.K.; Entezari, M.H.; Nancarrow, P. Chem. Eng. J. 2013, 231,365. |
| [64] | Li, B.; Hong, S.; Zhang, X.; Xiong, C.; Zhao, G.; Yang, Q.; Liu, H. Adv. Theory Simul. 2019, 2,1900155. |
/
| 〈 |
|
〉 |