

Synthesis and Properties of a Series of Pure Inorganic Ionic Liquids Based on Rare Earth Cations and Polyoxometalates
Received date: 2021-04-02
Online published: 2021-04-25
Supported by
National Natural Science Foundation of China(21872021); National Natural Science Foundation of China(21671033); National Natural Science Foundation of China(22071019)
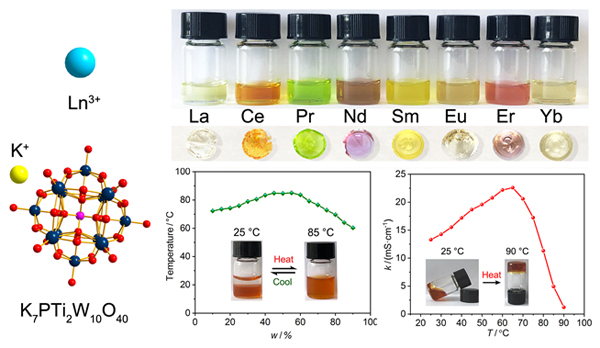
Ionic liquids (ILs) usually refer to ionic compounds that are liquid at a temperature of 100 ℃ and below. As salts, ionic liquids are composed of ions. So by selecting the corresponding cations, anions or ion combinations, they can be designed for specific applications. Various new types of ionic liquids have been prepared and discovered. Polyoxometalates (POMs) are a kind of metal-oxygen cluster compounds with rich composition and diverse structure. Compared with other common inorganic anions (such as PO43-, SO42-, CO32-,etc.), polyoxometalate anions have the characteristics of large ion radius and low charge density, which are a good anion choice for constructing ionic liquids. Most of the currently reported polyoxometalate ionic liquids are composed of organic cations and polyoxometalate anions, while pure inorganic ionic liquids composed of inorganic cations and polyoxometalate anions are rarely reported. In this work, we report a series of lanthanide- containing compounds based on a Ti-substituted Keggin-type polyoxometalate prepared by ion exchange, KLnH3PTi2W10O40•xH2O [Ln=La (1), Ce (2), Pr (3), Nd (4), Sm (5), Eu (6), Er (7), Yb (8)]. They were characterized by inductively coupled plasma emission spectrometry (ICP), thermal gravimetric analysis (TG), Fourier transform infrared (FT-IR) spectroscopy, powder X-ray diffraction (PXRD). Surprisingly, these inorganic ionic compounds behave as liquid with good fluidity in the temperature range of 4~65 ℃, and their conductivity are all higher than 10 mS•cm-1, showing ionic liquid behavior. Water is an indispensable component in their composition. After losing water, they become transparent and colored solids with amorphous structure. The study found that the conductivity of these ionic liquids change significantly with temperature. For example, when the temperature rises from room temperature to 65 ℃, the conductivity of Ce-containing ionic liquid gradually increases from 13.3 mS•cm-1 to 22.6 mS•cm-1. When the temperature rises from 65 ℃ to 90 ℃, the conductivity drops significantly, as low as 1.22 mS•cm-1 at 90 ℃. As far as we know, this kind of pure inorganic ionic liquid is very rare. The synthesis method of series ionic liquids is simple, easy to operate, and environmentally friendly. These properties make the series of ionic liquids have good machinability and potential applications in the fields of catalysis and phase separation.
He-Nan Wang , An-Ge Zhang , Zhong Zhang , Hong-Rui Tian , Qian Yue , Xue Zhao , Ying Lu , Shu-Xia Liu . Synthesis and Properties of a Series of Pure Inorganic Ionic Liquids Based on Rare Earth Cations and Polyoxometalates[J]. Acta Chimica Sinica, 2021 , 79(7) : 920 -924 . DOI: 10.6023/A21040130
| [1] | Hallett, J. P.; Welton, T. Chem. Rev. 2011, 111, 3508. |
| [2] | Lei, Z. G.; Chen, B. H.; Koo, Y. M.; MacFarlane, D. R. Chem. Rev. 2017, 117, 6633. |
| [3] | Rogers, R. D.; Seddon., K. R. Science 2003, 302, 792. |
| [4] | Kang, X. C.; Sun, X. F.; Han, B. X. Adv. Mater. 2016, 28, 1011. |
| [5] | Li, S. N.; Zhao, W. X.; Liu, Y. J.; Liu, Z. Q.; Ying, A. G. Chin. J. Org. Chem. 2020, 40, 1835. (in Chinese) |
| [5] | (李胜男, 赵雯辛, 刘玉静, 刘中秋, 应安国, 有机化学, 2020, 40, 1835.) |
| [6] | Kore, R.; Berton, P.; Kelley, S. P.; Aduri, P.; Katti, S. S.; Rogers, R. D. ACS Catal. 2017, 7, 7014. |
| [7] | Zhou, Z. H.; Chen, K. H.; He, L. N. Chin. J. Chem. 2019, 37, 1223. |
| [8] | Yao, W. H.; Wang, H. Y.; Pei, Y. C.; Chen, Y. H.; Li, Z. Y.; Wang, J. Y. RSC Adv. 2017, 7, 11297. |
| [9] | Sun, T. X.; Shen, X. H.; Chen, Q. D. Acta Phys.-Chim. Sin. 2015, 31, 32. (in Chinese) |
| [9] | (孙涛祥, 沈兴海, 陈庆德, 物理化学学报, 2015, 31, 32). |
| [10] | Bara, J. E.; Camper, D. E.; Gin, D. L.; Noble, R. D. Acc. Chem. Res. 2010, 43, 152. |
| [11] | Xing, H. B.; Liao, C.; Yang, Q. W.; Veith, G. M.; Kun, G. B.; Sun, X. G.; Ren, Q. L.; Hu, Y. S.; Dai, S. Angew. Chem. Int. Ed. 2014, 53, 2099. |
| [12] | Kang, S. S.; Fan, S. C.; Liu, Y.; Wei, Y. C.; Li, Y.; Fang, J. G.; Meng, C. Z. Acta Chim. Sinica 2019, 77, 647. (in Chinese) |
| [12] | (康树森, 范少聪, 刘岩, 魏彦存, 李营, 房金刚, 孟垂舟, 化学学报, 2019, 77, 647). |
| [13] | Miras, H. N.; Yan, J.; Long, D. L.; Cronin, L. Chem. Soc. Rev. 2012, 41, 7403. |
| [14] | Wang, S. S.; Yang, G. Y. Chem. Rev. 2015, 115, 4893. |
| [15] | Wei, Z. Y.; Chang, Y. L.; Yu, H.; Han, S.; Wei, Y. G. Acta Chim. Sinica 2020, 78, 725. (in Chinese) |
| [15] | (魏哲宇, 常亚林, 余焓, 韩生, 魏永革, 化学学报, 2020, 78, 725). |
| [16] | Leng, Y.; Wang, J.; Zhu, D. R.; Ren, X. Q.; Ge, H. Q.; Shen, L. Angew. Chem. Int. Ed. 2009, 48, 168. |
| [17] | Herrmann, S.; Kostrzewa, M.; Wierschem, A.; Streb, C. Angew. Chem. Int. Ed. 2014, 53, 13596. |
| [18] | Qiao, Y. X.; Hou, Z. S.; Li, H.; Hu, Y.; Feng, B.; Wang, X. R.; Hua, L.; Huang, Q. F. Green Chem. 2009, 11, 1955. |
| [19] | Dai, L. Y.; Yu, S. Y.; Shan, Y. K.; He, M. Y. Eur. J. Inorg. Chem. 2004,237. |
| [20] | Domaille, P. J.; Knoth, W. H. Inorg. Chem. 1983, 22, 818. |
| [21] | Bi, L. H.; Hussain, F.; Kortz, U.; Sadakane, M.; Dickman, M. H. Chem. Commun. 2004,1420. |
| [22] | Guan, W.; Yan, L. K.; Su, Z. M.; Liu, S. X.; Zhang, M.; Wang, X. H. Inorg. Chem. 2005, 44, 100. |
| [23] | Wang, X. N.; Liu, S. X.; Li, S. J.; Xie, R. H.; Zhang, X.; Liu, Y. W. Chem. J. Chinese Universities. 2013, 34, 1047. (in Chinese) |
| [23] | (王雪娜, 刘术侠, 李书军, 谢瑞红, 张鑫, 刘艺伟, 高等学校化学学报, 2013, 34, 1047). |
| [24] | Wang, C. L.; Liu, S. X.; Xie, L. H.; Ren, Y. H.; Liang, D. D.; Sun, C. Y.; Cheng, H. Y. Polyhedron 2007, 26, 3017. |
| [25] | Ichikawa, T.; Yoshio, M.; Hamasaki, A.; Taguchi, S.; Liu, F.; Zeng, X.-B.; Ungar, G.; Ohno, H.; Kato, T. J. Am. Chem. Soc. 2012, 134, 2634. |
| [26] | Wu, X. F.; Zhou, X. H.; Wu, Q. Y.; Yan, W. F. New J. Chem. 2016, 40, 7923. |
| [27] | Zheng, Q. G.; Liu, H.; Xia, Q.; Liu, Q. S.; Mou, L. Acta Phys.-Chim. Sin. 2017, 33, 736. (in Chinese) |
| [27] | (郑其格, 刘惠, 夏泉, 刘青山, 牟林, 物理化学学报, 2017, 33, 736.) |
| [28] | Wang, C.; Ying, J.; Mou, H. C.; Tian, A. X.; Wang, X. L. Inorg. Chem. Front. 2020, 7, 3882. |
| [29] | Qiao, Y. X.; Ma, W. B.; Theyssen, N.; Chen, C.; Hou, Z. S. Chem. Rev. 2017, 117, 6881. |
| [30] | Depuydt, D.; Van den Bossche, A.; Dehaen, W.; Binnemans, K. Chem. Commun. 2017, 53, 5271. |
| [31] | Blesic, M.; Gunaratne, H. Q. N.; Jacquemin, J.; Nockemann, P.; Olejarz, S.; Seddon, K. R.; Strauss, C. R. Green Chem. 2014, 16, 4115. |
| [32] | Fukaya, Y.; Ohno, H. Phys. Chem. Chem. Phys. 2013, 15, 4066. |
/
| 〈 |
|
〉 |