

Advances on Asymmetric Construction of Diarylmethine Stereocenters
Received date: 2021-07-26
Online published: 2021-09-18
Supported by
National Natural Science Foundation of China(21772078); National Natural Science Foundation of China(22071200); Science and Technology Department of Sichuan Province(2020JDRC0021); Fundamental Research Funds for the Central Universities(2682020CX55); Fundamental Research Funds for the Central Universities(2682021ZTPY011); Fundamental Research Funds for the Central Universities(XJ2021KJZK004)
Diarylmethine structure units are widely present in natural products and pharmaceuticals with important physiological and pharmacological activities. The enantioselective control of its stereogenic center, which is the most unique in this structure unit, has often become a difficulty and challenge during the research of total synthesis of natural products. Therefore, this field has attracted great interest to many organometallic chemists and synthetic organic chemists. In recent years, the construction methods of this stereocenter developed rapidly, and new reactions and reagents have emerged one after another. Some highly efficient catalysts have been invented, exhibiting unique catalytic activity and selectivity. In this review, according to the difference of reaction types, these methods can be divided into six types, such as asymmetric conjugate addition reaction (asymmetric 1,4-addition reactions involving aryl boronic acids, asymmetric 1,4-addition reactions involving aryl borates, enantioselective organocatalytic 1,4-addition reactions, asymmetric 1,4-conjugate addition induced by Evans chiral imide and asymmetric 1,6-conjugate addition of para-quinone methides), asymmetric allylation or propargylation of aromatic rings, transition metal-catalyzed asymmetric cross-coupling reaction, transition metal-catalyzed asymmetric C―H bond activation and functionalization, three-component reactions for asymmetric synthesis of 1,1-diaryl alkanes, asymmetric hydrogenation reactions for 1,1-diaryl alkanes, etc. This review aims to collect and summarize the asymmetric construction methods of diarylmethine stereogenic centers, and their applications in the total synthesis of natural products in the past decade. Finally, from the perspective of total synthesis, we further summarize and analyze the future development trend for the construction of diarylmethine chiral stereogenic centers, and encourage young generation to develop new methods and reagents that can avoid use of precious metals/catalysts and therefore are more efficient and environmentally friendly. More importantly, we hope that the developments of these practical methodologies can be further applied to asymmetric total synthesis of natural products and medicines, and eventually solve the source problem of these “useful molecules” with potential medicinal value.
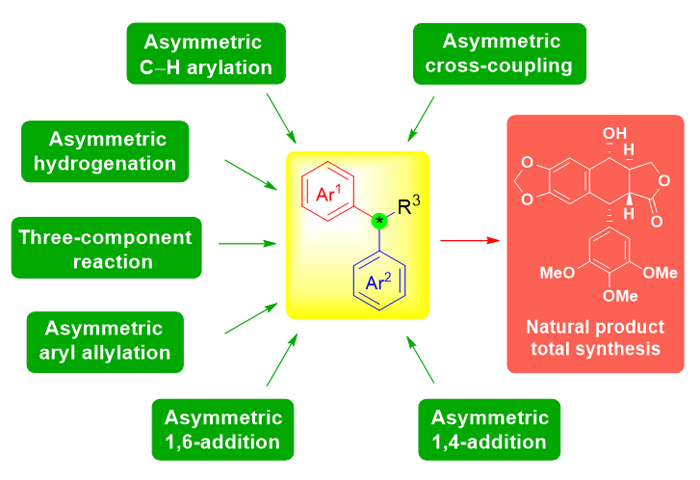
Yang Shang , Jian Xiao , Yawen Wang , Yu Peng . Advances on Asymmetric Construction of Diarylmethine Stereocenters[J]. Acta Chimica Sinica, 2021 , 79(11) : 1303 -1319 . DOI: 10.6023/A21070345
| [1] | Lindsay De Vane C.; Liston H. L.; Markowitz J. S. Clin Pharmacokinet. 2002, 41, 1247. |
| [2] | Hyttel J.; Larsen J. J. J. Neurochem. 1985, 44, 1615. |
| [3] | (a) Stähelin H. F.; von Wartburg A. Cancer Res. 1991, 51, 5. |
| [3] | (b) Liu Y.-Q.; Yang L.; Tian X. Curr. Bioact. Compd. 2007, 3, 37. |
| [4] | Sakai M.; Hayashi H.; Miyaura N. Organometallics 1997, 16, 4229. |
| [5] | Hayashi T.; Tokunaga N.; Okamoto K.; Shintani R. Chem. Lett. 2005, 34, 1480. |
| [6] | Paquin J.-F.; Defieber C.; Stephenson C. R. J.; Carreira E. M. J. Am. Chem. Soc. 2005, 127, 10850. |
| [7] | Yao J.; Yin L.; Shen Y.; Lu T.; Hayashi T.; Dou X. Org. Lett. 2018, 20, 6882. |
| [8] | Wang Z.-Q.; Feng C.-G.; Zhang S.-S.; Xu M.-H.; Lin G.-Q. Angew. Chem. Int. Ed. 2010, 49, 5780. |
| [9] | Lang F.; Chen G.; Li L.; Xing J.; Han F.; Cun L.; Liao J. Chem. Eur. J. 2011, 17, 5242. |
| [10] | Jumde V. R.; Iuliano A. Adv. Synth. Catal. 2013, 355, 3475. |
| [11] | He Q.; Xie F.; Fu G.; Quan M.; Shen C.; Yang G.; Gridnev I. D.; Zhang W. Org. Lett. 2015, 17, 2250. |
| [12] | Bao X.; Cao Y.-X.; Chu W.-D.; Qu H.; Du J.-Y.; Zhao X.-H.; Ma X.-Y.; Wang C.-T.; Fan C.-A. Angew. Chem. Int. Ed. 2013, 52, 14167. |
| [13] | (a) Wang J.; Wang M.; Cao P.; Jiang L.; Chen G.; Liao J. Angew. Chem. Int. Ed. 2014, 53, 6673. |
| [13] | (b) Han Z.; Wang Z.; Zhang X.; Ding K. Angew. Chem. Int. Ed. 2009, 48, 5345. |
| [14] | Lee A.; Kim H. J. Am. Chem. Soc. 2015, 137, 11250. |
| [15] | (a) Takatsu K.; Shintani R.; Hayashi T. Angew. Chem. Int. Ed. 2011, 50, 5548. |
| [15] | (b) Davies H. M.; Gregg T. M. Tetrahedron Lett. 2002, 43, 4951. |
| [16] | Shih J.-L.; Nguyen T. S.; May J. A. Angew. Chem. Int. Ed. 2015, 54, 9931. |
| [17] | Ming J.; Hayashi T. Org. Lett. 2016, 18, 6452. |
| [18] | Wu C.; Yue G.; Nielsen C. D.-T.; Xu K.; Hirao H.; Zhou J. J. Am. Chem. Soc. 2016, 138, 742. |
| [19] | Paras N. A.; MacMillan D. W. C. J. Am. Chem. Soc. 2002, 124, 7894. |
| [20] | Hong L.; Wang L.; Sun W.; Wong K.; Wang R. J. Org. Chem. 2009, 74, 6881. |
| [21] | Zhang H.; Liao Y.-H.; Yuan W.-C.; Zhang X.-M. Eur. J. Org. Chem. 2010, 3215. |
| [22] | Evans D. A.; Barotroli J.; Shih T. L. J. Am. Chem. Soc. 1981, 103, 2127. |
| [23] | Xiao J.; Cong X.-W.; Yang G.-Z.; Wang Y.-W.; Peng Y. Org. Lett. 2018, 20, 1651. |
| [24] | (a) Peng Y.; Luo Z.-B.; Zhang J.-J.; Luo L.; Wang Y.-W. Org. Biomol. Chem. 2013, 11, 7574. |
| [24] | (b) Zhang J.-J.; Yan C.-S.; Peng Y.; Luo Z.-B.; Xu X.-B.; Wang Y.-W. Org. Biomol. Chem. 2013, 11, 2498. |
| [24] | (c) Peng Y.; Xiao J.; Xu X.-B.; Duan S.-M.; Ren L.; Shao Y.-L.; Wang Y.-W. Org. Lett. 2016, 18, 5170. |
| [24] | (d) Luo Z.-B.; Wang Y.-W.; Peng Y. Org. Biomol. Chem. 2020, 18, 2054. |
| [25] | (a) Chu W.-D.; Zhang L.-F.; Bao X.; Zhao X.-H; Zeng C.; Du J.-Y.; Zhang G.-B.; Wang F.-X.; Ma X.-Y.; Fan C.-A. Angew. Chem. Int. Ed. 2013, 52, 9229. |
| [25] | (b) Lou Y.; Cao P.; Jia T.; Zhang Y.; Wang M.; Liao J. Angew. Chem. Int. Ed. 2015, 54, 12134. |
| [25] | (c) Li S.; Liu Y.; Huang B.; Zhou T.; Tao H.; Xiao Y.; Liu L.; Zhang J. ACS Catal. 2017, 7, 2805. |
| [25] | (d) Caruana L.; Kniep F.; Johansen T. K.; Poulsen P. H.; Jørgensen K. A. J. Am. Chem. Soc. 2014, 136, 15929. |
| [25] | (e) Li X.; Xu X.; Wei W.; Lin A.; Yao H. Org. Lett. 2016, 18, 428. |
| [25] | (f) Wen W.; Luo M.-J.; Yuan Y.; Liu J.-H.; Wu Z.-L.; Cai T.; Wu Z.-W.; Ouyang Q.; Guo Q.-X. Nat. Commun. 2020, 11, 5372. |
| [26] | (a) Falciola C. A.; Alexakis A. Angew. Chem. Int. Ed. 2007, 46, 2619; |
| [26] | (b) Kacprzynski M. A.; May T. L.; Kazane S. A. Hoveyda A. H. Angew. Chem. Int. Ed. 2007, 46, 4554. |
| [27] | Shintani R.; Takatsu K.; Takeda M.; Hayashi T. Angew. Chem. Int. Ed. 2011, 50, 8656. |
| [28] | Tian H.; Zhang P.; Peng F.; Yang H.; Fu H. Org. Lett. 2017, 19, 3775. |
| [29] | Shao L.; Hu X.-P. Org. Biomol. Chem. 2017, 15, 9837. |
| [30] | (a) Cheng R.; Sang X.; Gao X.; Zhang S.; Xue X.; Zhang X. Angew. Chem. Int. Ed. 2021, 60, 12386. |
| [30] | (b) Li X.; Gao X.; He C.; Zhang X. Org. Lett. 2021, 23, 1400. |
| [30] | (c) Weix D. J. Acc. Chem. Res. 2015, 48, 1767. |
| [30] | (d) Ackerman L. K. G.; Lovell M. M.; Weix D. J. Nature 2015, 524, 454. |
| [30] | (e) León T.; Correa A.; Martin R. Nature 2017, 545, 84. |
| [30] | (f) Li Z.-Q.; Wu D.; Ding C.; Yin G.-Y. CCS Chem. 2020, 2, 576. |
| [30] | (g) Belal M.; Li Z.-Q.; Lu X.-Q.; Yin G.-Y. Sci. China Chem. 2021, 64, 513. |
| [31] | (a) Sun D.; Ma G.; Zhao X.; Lei C.; Gong H. Chem. Sci. 2021, 12, 5253. |
| [31] | (b) Pan Q.; Ping Y.; Wang Y.; Guo Y.; Kong W. J. Am. Chem. Soc. 2021, 143, 10282. |
| [31] | (c) DeLano T. J.; Dibrell S. E.; Lacker C. R.; Pancoast A. R.; Poremba K. E.; Cleary L.; Sigman M. S.; Reisman S. E. Chem. Sci. 2021, 12, 7758. |
| [31] | (d) Fan P.; Lan Y.; Zhang C.; Wang C. J. Am. Chem. Soc. 2020, 142, 2180. |
| [31] | (e) Wang Z.; Yang Z.-P.; Fu G. C. Nat. Chem. 2021, 13, 236. |
| [32] | (a) Do H.-Q.; Chandrashekar E. R. R.; Fu G. C. J. Am. Chem. Soc. 2013, 135, 16288. |
| [32] | (b) Wilsily A.; Tramutola F.; Owston N. A.; Fu G. C. J. Am. Chem. Soc. 2012, 134, 5794. |
| [32] | (c) Binder J. T.; Cordier C. J.; Fu G. C. J. Am. Chem. Soc. 2012, 134, 17003. |
| [33] | Woods B. P.; Orlandi M.; Huang C.-Y.; Sigman M. S.; Doyle A. G. J. Am. Chem. Soc. 2017, 139, 5688. |
| [34] | (a) Poremba K. E.; Kadunce N. T.; Suzuki N.; Cherney A. H.; Reisman S. E. J. Am. Chem. Soc. 2017, 139, 5684. |
| [34] | (b) Cherney A. H.; Kadunce N. T.; Reisman S. E. J. Am. Chem. Soc. 2013, 135, 7442. |
| [35] | (a) Li B.; Aliyu M. A.; Gao Z.; Li T.; Dong W.; Li J.; Shi E.; Tang W. Org. Lett. 2020, 22, 4974. |
| [35] | (b) Huang K.-C.; Gopula B.; Kuo T.-S.; Chiang C.-W.; Wu P.-Y.; Henschke J. P.; Wu H.-L. Org. Lett. 2013, 15, 5730. |
| [36] | (a) Yue G.; Lei K.; Hirao H.; Zhou J. Angew. Chem. Int. Ed. 2015, 54, 6531. |
| [36] | (b) Qin X.; Lee M. W. Y.; Zhou J. Org. Lett. 2019, 21, 5990. For a review, see: |
| [36] | (c) Oxtoby L. J.; Gurak J. A. Jr.; Wisniewski S. R.; Eastgate M. D.; Engle K. M. Trends Chem. 2019, 1, 572. |
| [37] | Chen G.; Gong W.; Zhuang Z.; Andrä M. S.; Chen Y.-Q.; Hong X.; Yang Y.-F.; Liu T.; Houk K. N.; Yu J. Q. Science 2016, 353, 1023. |
| [38] | Zhang W.; Wu L.; Chen P.; Liu G. Angew. Chem. Int. Ed. 2019, 58, 6425. |
| [39] | Cheng X.; Lu H.; Lu Z. Nature Commun. 2019, 10, 3549. |
| [40] | Yamamoto E.; Hilton M. J.; Orlandi M.; Saini V.; Toste F. D.; Sigman M. S. J. Am. Chem. Soc. 2016, 138, 15877. |
| [41] | (a) Wu L.; Wang F.; Wan X.; Wang D.; Chen P.; Liu G. J. Am. Chem. Soc. 2017, 139, 2904. |
| [41] | (b) Wang D.; Wu L.; Wang F.; Wan X.; Chen P.; Lin Z.; Liu G. J. Am. Chem. Soc. 2017, 139, 6811. |
| [42] | Chen B.; Cao P.; Yin X.; Liao Y.; Jiang L.; Ye J.; Wang M.; Liao J. ACS Catal. 2017, 7, 2425. |
| [43] | Anthony D.; Lin Q.; Baudet J.; Diao T. Angew. Chem. Int. Ed. 2019, 58, 3198. |
| [44] | Sakurai S.; Matsumoto A.; Kano T.; Maruoka K. J. Am. Chem. Soc. 2020, 142, 19017. |
| [45] | Song S.; Zhu S.-F.; Yu Y.-B.; Zhou Q.-L. Angew. Chem. Int. Ed. 2013, 52, 1556. |
| [46] | Li Y.; Dong K.; Wang Z.; Ding K. Angew. Chem. Int. Ed. 2013, 52, 6748. |
| [47] | Nie H.; Zhu Y.; Hu X.; Wei Z.; Yao L.; Zhou G.; Wang P.; Jiang R.; Zhang S. Org. Lett. 2019, 21, 8641. |
| [48] | Xu B.; Li M.-L.; Zuo X.-D.; Zhu S.-F.; Zhou Q.-L. J. Am. Chem. Soc. 2015, 137, 8700. |
| [49] | Zhu D.-X.; Xia H.; Liu J.-G.; Chung L.-W.; Xu M.-H. J. Am. Chem. Soc. 2021, 143, 2608. |
| [50] | Guo Q. Chin. J. Org. Chem. 2019, 39, 2912. (in Chinese) |
| [50] | ( 郭庆君, 有机化学 2019, 39, 2912.) |
/
| 〈 |
|
〉 |