

Pd-Catalyzed One-Pot Synthesis of Difunctionalized o-Carboranes via Construction of B—C and B—Heteroatom Bonds※
Received date: 2021-12-29
Online published: 2022-02-07
Supported by
National Natural Science Foundation of China(92056106); Hong Kong Research Grants Council(14305018)
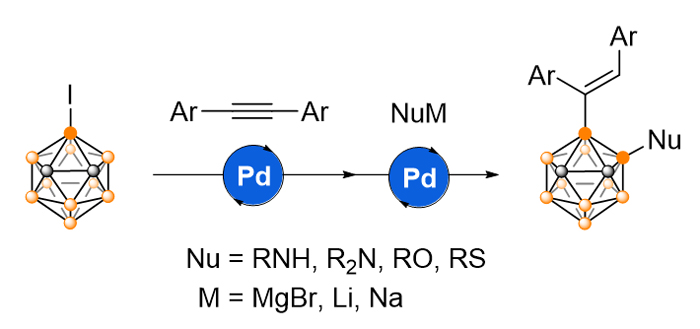
Icosahedral carboranes are carbon-boron molecular clusters, sharing many features with benzene such as aromaticity, high thermal and chemical stability. On the other hand, carboranes have their own unique characteristics like spherical geometry and three-dimensional electronic delocalization. These properties render carboranes unique building blocks for various applications ranging from versatile ligands to functional materials to medicine. In this regard, functionalization of carboranes, particularly regioselective functionalization of cage B-vertexes has recently received much attention. Based on our recently developed Pd-catalyzed iodine-migration on o-carborane cage, a Pd-catalyzed regioselective difunctionalization of 3-iodo-o-carborane in a one-pot manner has been achieved to afford a series of 3-alkenyl-4-Nu-o-carboranes (Nu=arylamino, alkoxyl, alkyl and arylthio) in 47%~99% yields. This protocol combines the sequential activation of cage B(3)—I and B(4)—H bonds by Pd migration, as well as further Pd-catalyzed transformation of B(4)—I bond, leading to the construction of B—C and B—Heteroatom bonds. A general procedure for the synthesis of 3-alkenyl-4-Nu-o-carboranes is described as follows: to a tetrahydrofuran (THF) solution (1 mL) of NuH (1.0 mmol) was added base (1.0 mmol) at 0 ℃ under an atmosphere of dry nitrogen. The reaction mixture was stirred for another 10 min to obtain the NuM solution (NuM=ArNHMgBr, base=EtMgBr; NuM=Ar2NLi, base=nBuLi). Another oven-dried Schlenk flask equipped with a stir bar was charged with 3-iodo-o-carborane (1, 27 mg, 0.1 mmol), Pd(PPh3)4 (12 mg, 0.01 mmol), diphenylacetylene (89 mg, 0.5 mmol) and dry toluene (1 mL) under an atmosphere of dry nitrogen. The flask was closed, and stirred at 80 ℃ for 72 h. Then, the resulting solution was cooled to 0 ℃, to which was slowly added NuM (0.15 mmol) (NuM=ArNHMgBr, Ar2NLi, tBuONa and RSNa). The reaction mixture was warmed to room temperature, and stirred at 80 ℃ for 24 h. After quenching with water (1 mL) and extraction with ethyl acetate (5 mL×3), the organic portions were combined and concentrated to dryness in vacuo. The residue was subjected to flash column chromatography on silica gel (300~400 mesh) using n-hexane as eluent to give the product.
Key words: carborane; palladium catalysis; metal migration; B—C bond; B—Heteroatom bond
Yixiu Ge , Zaozao Qiu , Zuowei Xie . Pd-Catalyzed One-Pot Synthesis of Difunctionalized o-Carboranes via Construction of B—C and B—Heteroatom Bonds※[J]. Acta Chimica Sinica, 2022 , 80(4) : 432 -437 . DOI: 10.6023/A21120597
| [1] | Carboranes, 3rd ed., Ed.: Grimes, R. N., Elsevier, Oxford, U. K., 2016. |
| [2] | For selected reviews in applications in medicine, see: (a) Hawthorne, M. F.; Maderna, A. Chem. Rev. 1999, 99, 3421. |
| [2] | (b) Scholz, M.; Hey-Hawkins, E. Chem. Rev. 2011, 111, 7035. |
| [2] | (c) Stockmann, P.; Gozzi, M.; Kuhnert, R.; Sárosi, M. B.; Hey-Hawkins, E. Chem. Soc. Rev. 2019, 48, 3497. |
| [3] | (a) Yang, X.; Jiang, W.; Knobler, C. B.; Hawthorne, M. F. J. Am. Chem. Soc. 1992, 114, 9719. |
| [3] | (b) Jude, H.; Disteldorf, H.; Fischer, S.; Wedge, T.; Hawkridge, A. M.; Arif, A. M.; Hawthorne, M. F.; Muddiman, D. C.; Stang, P. J. J. Am. Chem. Soc. 2005, 127, 12131. |
| [3] | (c) Koshino, M.; Tanaka, T.; Solin, N.; Suenaga, K.; Isobe, H.; Nakamura, E. Science 2007, 316, 853. |
| [3] | (d) Dash, B. P.; Satapathy, R.; Gaillard, E. R.; Maguire, J. A.; Hosmane, N. S. J. Am. Chem. Soc. 2010, 132, 6578. |
| [3] | (e) Bauduin, P.; Prevost, S.; Farràs, P.; Teixidor, F.; Diat, O.; Zemb, T. Angew. Chem., Int. Ed. 2011, 50, 5298. |
| [3] | (f) Cioran, A. M.; Musteti, A. D.; Teixidor, F.; Krpetić, Z?.; Prior, I. A.; He, Q.; Kiely, C. J.; Brust, M.; Viñas, C. J. Am. Chem. Soc. 2012, 134, 212. |
| [3] | (g) Brusselle, D.; Bauduin, P.; Girard, L.; Zaulet, A.; Viñas, C.; Teixidor, F.; Ly, I.; Diat, O. Angew. Chem., Int. Ed. 2013, 52, 12114. |
| [3] | (h) Schwartz, J. J.; Mendoza, M. A.; Wattanatorn, N.; Zhao, Y.; Nguyen, V. T.; Spokoyny, A. M.; Mirkin, C. A.; Baše, T.; Weiss, P. S. J. Am. Chem. Soc. 2016, 138, 5957. |
| [3] | (i) Fisher, S. P.; Tomich, A. W.; Lovera, S. O.; Kleinsasser, J. F.; Guo, J.; Asay, M. J.; Nelson, H. M.; Lavallo, V. Chem. Rev. 2019, 119, 8262. |
| [3] | (j) Cui, P.-F.; Lin, Y.-J.; Li, Z.-H.; Jin, G.-X. J. Am. Chem. Soc. 2020, 142, 8532. |
| [3] | (k) Cui, P.-F.; Liu, X.-R.; Guo, S.-T.; Lin, Y.-J.; Jin, G.-X. J. Am. Chem. Soc. 2021, 143, 5099. |
| [4] | (a) Núñez, R.; Tarrés, M.; Ferrer-Ugalde, A.; de Biani, F. F.; Teixidor, F. Chem. Rev. 2016, 116, 14307. |
| [4] | (b) Fisher, S. P.; Tomich, A. W.; Lovera, S. O.; Kleinsasser, J. F.; Guo, J.; Asay, M. J.; Nelson, H. M.; Lavallo, V. Chem. Rev. 2019, 119, 8262. |
| [4] | (c) Keener, M.; Hunt, C.; Carroll, T. G.; Kampel, V.; Dobrovetsky, R.; Hayton, T. W.; Ménard, G. Nature 2020, 577, 652. |
| [4] | (d) Jung, D.; Saleh, L. M. A.; Berkson, Z. J.; El-Kady, M. F.; Hwang, J. Y.; Mohamed, N.; Wixtrom, A. I.; Titarenko, E.; Shao, Y.; McCarthy, K.; Guo, J.; Martini, I. B.; Kraemer, S.; Wegener, E. C.; Saint-Cricq, P.; Ruehle, B.; Langeslay, R. R.; Delferro, M.; Brosmer, J. L.; Hendon, C. H.; Gallagher-Jones, M.; Rodriguez, J.; Chapman, K. W.; Miller, J. T.; Duan, X.; Kaner, R. B.; Zink, J. I.; Chmelka, B. F.; Spokoyny, A. M. Nat. Mater. 2018, 17, 341. |
| [4] | (e) Nu?n?ez, R.; Romero, I.; Teixidor, F.; Vin?as, C. Chem. Soc. Rev. 2016, 45, 5147. |
| [4] | (f) Mukherjee, S.; Thilagar, P. Chem. Commun. 2016, 52, 1070. |
| [4] | (g) Wei, X.; Zhu, M.-J.; Cheng, Z.; Lee, M.; Yan, H.; Lu, C.; Xu, J.-J. Angew. Chem., Int. Ed. 2019, 58, 3162. |
| [5] | For selected reviews in applications in organometallic/coordination chemistry, see: (a) Xie, Z.. Coord. Chem. Rev. 2002, 231, 23. |
| [5] | (b) Yao, Z.-J.; Jin, G.-X. Coord. Chem. Rev. 2013, 257, 2522. |
| [5] | (c) Fisher, S. P.; Tomich, A. W.; Guo, J.; Lavallo, V. Chem. Commun. 2019, 55, 1684. |
| [6] | (a) Grimes, R. N. Dalton Trans. 2015, 44, 5939. |
| [6] | (b) Olid, D.; Núñez, R.; Viñas, C.; Teixidor, F. Chem. Soc. Rev. 2013, 42, 3318. |
| [6] | (c) Dziedzic, R. M.; Spokoyny, A. M. Chem. Commun. 2019, 55, 430. |
| [6] | (d) Qiu, Z.; Ren, S.; Xie, Z. Acc. Chem. Res. 2011, 44, 299. |
| [6] | (e) Zhao, D.; Xie, Z. Coord. Chem. Rev. 2016, 314, 14. |
| [6] | (f) Zhang, X.; Yan, H. Coord. Chem. Rev. 2019, 378, 466. |
| [6] | (g) Mu, W.; Cheng, R.; Shang, Y.; He, R.; Li, D.; Fu, M. Chin. J. Org. Chem. 2018, 38, 1327. (in Chinese) |
| [6] | (母伟花, 程瑞姣, 尚英伟, 贺仁泽, 李冬丽, 傅冕, 有机化学, 2018, 38, 1327.) |
| [6] | (h) Xu, X.; Cheng, R.; Qiu, Z.; Pan, C. Chin. J. Org. Chem. 2018, 38, 3078. (in Chinese) |
| [6] | (许新彬, 程若飞, 邱早早, 潘成岭, 2018, 38, 3078.) |
| [6] | (i) Mu, W.; Ma, Y.; Fang, D.; Wang, R.; Zhang, H. Acta Chim. Sinica 2018, 76, 55. (in Chinese) |
| [6] | (母伟花, 马瑶, 方德彩, 王蓉, 张海娜, 化学学报, 2018, 76, 55.) |
| [7] | (a) Zakharkin, L. I.; Stanko, V. I.; Brattstev, V. A.; Chapovskii, Y. A.; Struchkov, Y. T. Russ. Chem. Bull. 1963, 12, 1911. |
| [7] | (b) Zakharkin, L. I.; Stanko, V. I.; Brattsev, V. A.; Chapovskii, Y. A.; Okhlobystin, O. Y. Russ. Chem. Bull. 1963, 12, 2074. |
| [7] | (c) Heying, T. L.; Ager, J. W.; Clark, S. L.; Mangold, D. J.; Goldstein, H. L.; Hillman, M.; Polak, R. J.; Szymanski, J. W. Inorg. Chem. 1963, 2, 1089. |
| [7] | (d) Fein, M. M.; Bobinski, J.; Mayes, N.; Schwartz, N.; Cohen, M. S. Inorg. Chem. 1963, 2, 1111. |
| [8] | (a) Quan, Y.; Qiu, Z.; Xie, Z. Chem. Eur. J. 2018, 24, 2795. |
| [8] | (b) Quan, Y.; Xie, Z. Chem. Soc. Rev. 2019, 48, 3660. |
| [8] | (c) Au, Y. K.; Xie, Z. Bull. Chem. Soc. Jpn. 2021, 94, 879. |
| [8] | (d) Qiu, Z.; Xie, Z. Acc. Chem. Res. 2021, 54, 4065. |
| [8] | (e) Lian, L.; Yin, J.; Lin, C.; Ye, K.; Yuan, Y. Chin. J. Org. Chem. 2021, 41, 3249. (in Chinese) |
| [8] | (连凌翔, 尹静怡, 林彩霞, 叶克印, 袁耀锋, 有机化学, 2021, 41, 3249.) |
| [8] | (f) Zhang, H.; Qiu, Z.; Xie, Z. Chin. J. Org. Chem. 2020, 40, 3203. (in Chinese) |
| [8] | (张慧芳, 邱早早, 谢作伟, 有机化学, 2020, 40, 3203.) |
| [8] | (g) Liu, X.-R.; Cui, P.-F.; Guo, S.-T.; Yuan, R.-Z.; Jin, G.-X. Inorg. Chem. Front. 2021, 8, 4349. |
| [9] | Zhang, Z.-Y.; Zhang, X.; Yuan, J.; Yue, C.-D.; Meng, S.; Chen, J.; Yu, G.-A.; Che, C.-M. Chem. Eur. J. 2020, 26, 5037. |
| [10] | Cheng, R.; Qiu, Z.; Xie, Z. Nat. Commun. 2017, 8, 14827. |
| [11] | (a) Lyu, H.; Quan, Y.; Xie, Z. Angew. Chem., Int. Ed. 2016, 55, 11840. |
| [11] | (b) Guo, S.-T.; Cui, P.-F.; Yuan, R.-Z.; Jin, G.-X. Chem. Commun. 2021, 57, 2412. |
| [12] | (a) Li, C.-X.; Zhang, H.-Y.; Wong, T.-Y.; Cao, H.-J.; Yan, H.; Lu, C.-S. Org. Lett. 2017, 19, 5178. |
| [12] | (b) Cao, K.; Xu, T.-T.; Wu, J.; Jiang, L.; Yang, J. Chem. Commun. 2016, 52, 11446. |
| [13] | (a) Dziedzic, R. M.; Martin, J. L.; Axtell, J. C.; Saleh, L. M. A.; Ong, T.-C.; Yang, Y.-F.; Messina, M. S.; Rheingold, A. L.; Houk, K. N.; Spokoyny, A. M. J. Am. Chem. Soc. 2017, 139, 7729. |
| [13] | (b) Cui, C. X.; Zhang, J.; Qiu, Z.; Xie, Z. Dalton Trans. 2020, 49, 1380. |
| [13] | (c) Au, Y. K.; Lyu, H.; Quan, Y.; Xie, Z. J. Am. Chem. Soc. 2020, 142, 6940. |
| [14] | (a) Lyu, H.; Quan, Y.; Xie, Z. J. Am. Chem. Soc. 2016, 138, 12727. |
| [14] | (b) Au, Y. K.; Zhang, J.; Quan, Y.; Xie, Z. J. Am. Chem. Soc. 2021, 143, 4148. |
| [15] | (a) Han, G. U.; Baek, Y.; Lee, K.; Shin, S.; Chan Noh, H.; Lee, P. H. Org. Lett. 2021, 23, 416. |
| [15] | (b) Baek, Y.; Kim, S.; Son, J.-Y.; Lee, K.; Kim, D.; Lee, P. H. ACS Catal. 2019, 9, 10418. |
| [16] | Chen, Y.; Quan, Y.; Xie, Z. Chem. Commun. 2020, 56, 12997. |
| [17] | (a) Au, Y. K.; Lyu, H.; Quan, Y.; Xie, Z. Chin. J. Chem. 2020, 38, 383. |
| [17] | (b) Au, Y. K.; Lyu, H.; Quan, Y.; Xie, Z. J. Am. Chem. Soc. 2019, 141, 12855. |
| [17] | (c) Cheng, B.; Chen, Y.; Zhou, P.; Xie, Z. Chem. Commun. 2022, DOI: 10.1039/D1CC05936J. |
| [17] | (d) Cao, H.-J.; Chen, M.; Sun, F.; Zhao, Y.; Lu, C.; Zhang, X.; Shi, Z.; Yan, H. ACS Catal. 2021, 11, 14047. |
| [18] | (a) Li, J.; Logan, C. F.; Jones, M. Inorg. Chem. 1991, 30, 4866. |
| [18] | (b) Viñas, C.; Barberà, G.; Oliva, J. M.; Teixidor, F.; Welch, A. J.; Rosair, G. M. Inorg. Chem. 2001, 40, 6555. |
| [19] | Sevryugina, Y.; Julius, R.; Hawthorne, M. F. Inorg. Chem. 2010, 49, 10627. |
| [20] | Dziedzic, R. M.; Saleh, L. M. A.; Axtell, J. C.; Martin, J. L.; Stevens, S. L.; Timothy Royappa, A.; Rheingold, A. L.; Spokoyny, A. M. J. Am. Chem. Soc. 2016, 138, 9081. |
| [21] | Kabytaev, K. Z.; Everett, T. A.; Safronov, A. V.; Sevryugina, Y. V.; Jalisatgi, S. S.; Hawthorne, M. F. Eur. J. Inorg. Chem. 2013, 2488. |
| [22] | (a) Liu, D.; Dang, L.; Sun, Y.; Chan, H.-S.; Lin, Z.; Xie, Z. J. Am. Chem. Soc. 2008, 130, 16103. |
| [22] | (b) Eleazer, B. J.; Smith, M. D.; Popov, A. A.; Peryshkov, D. V. Chem. Sci. 2017, 8, 5399. |
| [22] | (c) Dziedzic, R. M.; Axtell, J. C.; Rheingold, A. L.; Spokoyny, A. M. Org. Process Res. Dev. 2019, 23, 1638. |
| [22] | (d) Dziedzic, R. M.; Martin, J. L.; Axtell, J. C.; Saleh, L. M. A.; Ong, T.-C.; Yang, Y.-F.; Messina, M. S.; Rheingold, A. L.; Houk, K. N.; Spokoyny, A. M. J. Am. Chem. Soc. 2017, 139, 7729. |
| [22] | (e) Lyu, H.; Zhang, J.; Yang, J.; Quan, Y.; Xie, Z. J. Am. Chem. Soc. 2019, 141, 4219. |
| [22] | (f) Guo, C.; Qiu, Z.; Xie, Z. ACS Catal. 2021, 11, 2134. |
| [23] | (a) Ge, Y.; Zhang, J.; Qiu, Z.; Xie, Z. Angew. Chem., Int. Ed. 2020, 59, 4851. |
| [23] | (b) Ge, Y.; Qiu, Z.; Xie, Z. Chem. Commun. 2021, 57, 8071. |
| [23] | (c) Ge, Y.; Zhang, J.; Qiu, Z.; Xie, Z. Dalton Trans. 2021, 50, 1766. |
/
| 〈 |
|
〉 |