

Recognition and Luminescence Properties of N^C^N Pt(II) Complexes with Macrocyclic Host Cucurbit[10]uril
Received date: 2022-02-20
Online published: 2022-04-01
Supported by
National Natural Science Foundation of China(21871216); National Natural Science Foundation of China(21901194)
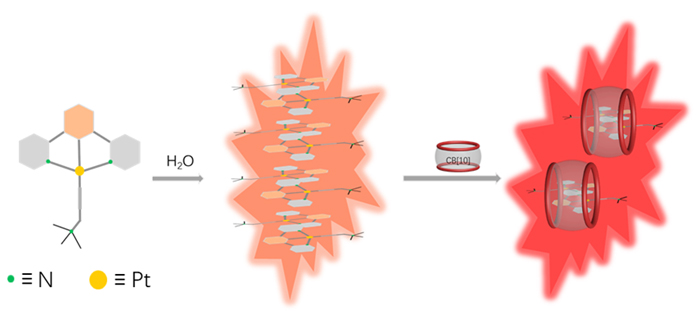
Transition metal complexes have been widely studied due to their unique photophysical and chemical properties. In order to further explore the influence of host-guest interaction on luminescent property of Pt complexes, three different substituted water-soluble Pt complexes were designed and synthesized. Cucurbit[10]uril (CB[10]), with the largest cavity among the cucurbit[n]uril family, was selected as the host molecule. The 1H NMR titration experiments proved CB[10] binded three guests with 1∶2 stoichiometry, and the 1,3-di(2-pyridyl)benzene (N^C^N) ligand part on the guest molecule was encapsulated antiparallelly in the cavity of CB[10] in a head-to-tail manner. Mass spectrometry characterization further proved the binding between host and guest with 1∶2 stoichiometry. The low-energy absorption peaks of N^C^N Pt(II) complexes at >400 nm attributed to metal-metal-to-ligand charge transfer (MMLCT) were enhanced upon binding with CB[10]. Compared to free [2,6-di(2-pyridinyl)phenyl][3-(trimethylammonio)-1-propyn-1-yl]platinum(II) chloride (Pt-1), the 3IL emission intensity of its complex with CB[10] was enhanced dominantly. The [methyl 3,5-di(2-pyridyl)benzoato][3-(tri- methylammonio)-1-propyn-1-yl]platinum(II) chloride (Pt-2) showed visible red phosphorescence emission at 648 nm which was attributed to 3MMLCT in aqueous solution. Interestingly, binding with CB[10] led to a red-shift to 667 nm, which was closer to near-infrared emission. Besides, the phosphorescence lifetime is enhanced by 1.5 times (14.64 to 23.74 μs), and the quantum yield is enhanced by 15.6 times (2.7% to 42%) upon the host-guest complexation. The luminescent property of [4-methoxy-2,6-di(2-pyridinyl)phenyl][3-(trimethylammonio)-1-propyn-1-yl]platinum(II) chloride (Pt-3) was similar to that of Pt-2. Further research on the luminescence changes of Pt complexes before and after grinding in solid state indicated that the host-guest complex showed bigger red-shift of emission wavelength than free guest. The above results showed that the host-guest interactions based on CB[10] could shorten the distance between the platinum atoms of the two included guest molecules, thus enhancing metal-metal interaction and π-π interaction of the host-guest complexes.
Shimin Zhu , Xin Huang , Xie Han , Simin Liu . Recognition and Luminescence Properties of N^C^N Pt(II) Complexes with Macrocyclic Host Cucurbit[10]uril[J]. Acta Chimica Sinica, 2022 , 80(8) : 1066 -1070 . DOI: 10.6023/A22020078
| [1] | (a) Wong, K. M.-C.; Yam, V. W.-W. Acc. Chem. Res. 2011, 44, 424. |
| [1] | (b) Lai, P.-N.; Brysacz, C. H.; Alam, M. K.; Ayoub, N. A.; Gray, T. G.; Bao, J.; Teets, T. S. J. Am. Chem. Soc. 2018, 140, 10198. |
| [2] | (a) Mauro, M.; Aliprandi, A.; Septiadi, D.; Kehr, N. S.; De Cola, L. Chem. Soc. Rev. 2014, 43, 4144. |
| [2] | (b) Chan, A. K.-W.; Ng, M.; Wong, Y.-C.; Chan, M.-Y.; Wong, W.-T.; Yam, V. W.-W. J. Am. Chem. Soc. 2017, 139, 10750. |
| [2] | (c) Lin, J.; Zou, C.; Zhang, X.; Gao, Q.; Suo, S.; Zhuo, Q.; Chang, X.; Xie, M.; Lu, W. Dalton Trans. 2019, 48, 10417. |
| [2] | (d) Allampally, N. K.; Bredol, M.; Strassert, C. A.; De Cola, L. Chem.-Eur. J. 2014, 20, 16863. |
| [3] | Li, K.; Ming Tong, G. S.; Wan, Q.; Cheng, G.; Tong, W.-Y.; Ang, W.-H.; Kwong, W.-L.; Che, C.-M. Chem. Sci. 2016, 7, 1653. |
| [4] | Chow, P.-K.; To, W.-P.; Low, K.-H.; Che, C.-M. Chem.-Asian J. 2014, 9, 534. |
| [5] | Wang, D.; Chen, Y.; Liu, X.; Bian, J.; Yin, X.; Teng, M.; Rong, M.; Wang, Z. Chin. J. Inorg. Chem. 2021, 37, 33. (in Chinese) |
| [5] | (王登强, 陈宇, 刘小庆, 卞健健, 尹新颖, 滕明瑜, 戎梅竹, 汪正良, 无机化学学报, 2021, 37, 33.) |
| [6] | Xu, G; Li, J.; Chen, Z. Acta Chim. Sinica 2014, 72, 667. (in Chinese) |
| [6] | (徐广涛, 李佳, 陈忠宁, 化学学报, 2014, 72, 667.) |
| [7] | Zhang, S.; Wang, S.; Zhou, X.; Zhang, H.; Shao, S.; Li, C. Acta Chim. Sinica 2008, 66, 841. (in Chinese) |
| [7] | (张首才, 王嵩, 周欣, 张红星, 邵琛, 李传碧, 化学学报, 2008, 66, 841.) |
| [8] | (a) Sun, C.-Y.; To, W.-P.; Hung, F.-F.; Wang, X.-L.; Su, Z.-M.; Che, C.-M. Chem. Sci. 2018, 9, 2357. |
| [8] | (b) Li, Z.; Han, Y.; Gao, Z.; Wang, F. ACS Catal. 2017, 7, 4676. |
| [9] | (a) Baggaley, E.; Botchway, S. W.; Haycock, J. W.; Morris, H.; Sazanovich, I. V.; Williams, J. A. G.; Weinstein, J. A. Chem. Sci. 2014, 5, 879. |
| [9] | (b) Ouyang, C.; Li, Y.; Rees, T. W.; Liao, X.; Jia, J.; Chen, Y.; Zhang, X.; Ji, L.; Chao, H. Angew. Chem., nt. Ed. 2021, 60, 4150. |
| [9] | (c) Law, A. S.-Y.; Lee, L. C.-C.; Lo, K. K.-W.; Yam, V. W.-W. J. Am. Chem. Soc. 2021, 143, 5396. |
| [10] | (a) Scoditti, S.; Dabbish, E.; Russo, N.; Mazzone, G.; Sicilia, E. Inorg. Chem. 2021, 60, 10350. |
| [10] | (b) Ramu, V.; Gautam, S.; Garai, A.; Kondaiah, P.; Chakravarty, A. R. Inorg. Chem. 2018, 57, 1717. |
| [11] | (a) Summa, G. M.; Scott, B. A. Inorg. Chem. 1980, 19, 1079. |
| [11] | (b) Yip, H.-K.; Cheng, L.-K.; Cheung, K.-K.; Che, C.-M. J. Chem. Soc., Dalton Trans. 1993, 2933. |
| [12] | (a) Yam, V. W.-W.; Tang, R. P.-L.; Wong, K. M.-C.; Cheung, K.-K. Organometallics 2001, 20, 4476. |
| [12] | (b) Du, P.; Schneider, J.; Jarosz, P.; Eisenberg, R. J. Am. Chem. Soc. 2006, 128, 7726. |
| [12] | (c) Yang, Z.; Tian, Y.; Li, Z.; Ao, L.; Gao, Z.; Wang, F. Acta Polym. Sin. 2017, 48, 121. (in Chinese) |
| [12] | (杨支帅, 田玉奎, 李子健, 敖雷, 高宗春, 汪峰, 高分子学报, 2017, 48, 121.) |
| [13] | (a) Yam, V. W.-W.; Wong, K. M.-C.; Zhu, N. J. Am. Chem. Soc. 2002, 124, 6506. |
| [13] | (b) Yam, V. W.-W.; Chan, K. H.-Y.; Wong, K. M.-C.; Zhu, N. Chem.-Eur. J. 2005, 11, 4535. |
| [14] | Williams, J. A. G. Chem. Soc. Rev. 2009, 38, 1783. |
| [15] | (a) Hu, S.-J.; Guo, X.-Q.; Zhou, L.-P.; Yan, D.-N.; Cheng, P.-M.; Cai, L.-X.; Li, X.-Z.; Sun, Q.-F. J. Am. Chem. Soc. 2022, 144, 4244. |
| [15] | (b) Gemen, J.; Ahrens, J.; Shimon, L. J. W.; Klajn, R. J. Am. Chem. Soc. 2020, 142, 17721. |
| [15] | (c) Benson, C. R.; Kacenauskaite, L.; VanDenburgh, K. L.; Zhao, W.; Qiao, B.; Sadhukhan, T.; Pink, M.; Chen, J.; Borgi, S.; Chen, C.-H.; Davis, B. J.; Simon, Y. C.; Raghavachari, K.; Laursen, B. W.; Flood, A. H. Chem 2020, 6, 1978. |
| [16] | (a) Liu, S.; Zavalij, P. Y.; Isaacs, L. J. Am. Chem. Soc. 2005, 127, 16798. |
| [16] | (b) Yang, X.; Liu, F.; Zhao, Z.; Liang, F.; Zhang, H.; Liu, S. Chin. Chem. Lett. 2018, 29, 1560. |
| [16] | (c) Tian, X.; Zuo, M.; Niu, P.; Wang, K.; Hu, X. Chin. J. Org. Chem. 2020, 40, 1823. (in Chinese) |
| [16] | (田雪琪, 左旻瓒, 牛蓬勃, 王开亚, 胡晓玉, 有机化学, 2020, 40, 1823.) |
| [17] | (a) Anis-Ul-Haque, K. M.; Woodward, C. E.; Day, A. I.; Wallace, L. Inorg. Chem. 2020, 59, 3942. |
| [17] | (b) Luis, E. T.; Day, A. I.; König, B.; Beves, J. E. Inorg. Chem. 2020, 59, 9135. |
| [17] | (c) Zhang, Y.; Liu, M.; Karatchevtseva, I.; Price, J. R.; Tao, Z.; Wei, G. New J. Chem. 2020, 44, 18208. |
| [18] | (a) Kuang, S.; Hu, Z.; Zhang, H.; Zhang, X.; Liang, F.; Zhao, Z.; Liu, S. Chem. Commun. 2018, 54, 2169. |
| [18] | (b) Deng, Y.; Yin, H.; Zhao, Z.; Wang, R.; Liu, S. Supramol. Chem. 2018, 30, 706. |
| [19] | Hu, Z.; Sun, D.; Han, X.; Liu, S. Chin. J. Org. Chem. 2020, 40, 1361. (in Chinese) |
| [19] | (胡智雄, 孙冬冬, 韩勰, 刘思敏, 有机化学, 2020, 40, 1361.) |
| [20] | (a) Chen, Y.; Li, K.; Lu, W.; Chui, S. S.-Y.; Ma, C.-W.; Che, C.-M. Angew. Chem., Int. Ed. 2009, 48, 9909. |
| [20] | (b) Williams, J. A. G.; Beeby, A.; Davies, E. S.; Weinstein, J. A.; Wilson, C. Inorg. Chem. 2003, 42, 8609. |
| [20] | (c) Cárdenas, D. J.; Echavarren, A. M.; Ramírez de Arellano, M. C. Organometallics 1999, 18, 3337. |
| [20] | (d) Wang, Z.; Turner, E.; Mahoney, V.; Madakuni, S.; Groy, T.; Li, J. Inorg. Chem. 2010, 49, 11276. |
| [21] | Wan, Q.; Xiao, X.-S.; To, W.-P.; Lu, W.; Chen, Y.; Low, K.-H.; Che, C.-M. Angew. Chem., nt. Ed. 2018, 57, 17189. |
| [22] | (a) Chen, Y.; Lu, W.; Che, C.-M. Organometallics 2013, 32, 350. |
| [22] | (b) Ai, Y.; Chan, M. H.-Y.; Chan, A. K.-W.; Ng, M.; Li, Y.; Yam, V. W.-W. Proc. Natl. Acad. Sci. U. S. A. 2019, 116, 13856. |
| [23] | Li, B.; Li, Y.; Chan, M. H.-Y.; Yam, V. W.-W. J. Am. Chem. Soc. 2021, 143, 21676. |
| [24] | Xie, M.; Lu, W. Dalton Trans. 2019, 48, 1275. |
| [25] | Zhu, S.; Hu, J.; Zhai, S.; Wang, Y.; Xu, Z.; Liu, R.; Zhu, H. Inorg. Chem. Front. 2020, 7, 4677. |
| [26] | Han, X.; Sun, D.; Tang, S.; Wu, Y.; Wang, L.; Zhang, X.; Liu, S. J. Mater. Chem. C 2021, 9, 17307. |
| [27] | (a) Kozhevnikov, V. N.; Donnio, B.; Bruce, D. W. Angew. Chem., nt. Ed. 2008, 47, 6286. |
| [27] | (b) Choi, S. J.; Kuwabara, J.; Nishimura, Y.; Arai, T.; Kanbara, T. Chem. Lett. 2011, 41, 65. |
| [27] | (c) Abe, T.; Itakura, T.; Ikeda, N.; Shinozaki, K. Dalton Trans. 2009, 711. |
| [27] | (d) Zhang, X.-P.; Mei, J.-F.; Lai, J.-C.; Li, C.-H.; You, X.-Z. J. Mater. Chem. C 2015, 3, 2350. |
/
| 〈 |
|
〉 |