

Nitrogen-doped Carbon Pyrolyzed from ZIF-8 for Electrocatalytic Oxygen Reduction to Hydrogen Peroxide
Received date: 2022-01-16
Online published: 2022-04-27
Supported by
National Key Research and Development Project of China(2016YFB0301602); State Key Laboratory of Catalytic Materials and Reaction Engineering (RIPP, SINOPEC); National Natural Science Foundation of China(21872035); Science and Technology Commission of Shanghai Municipality(19DZ2270100)
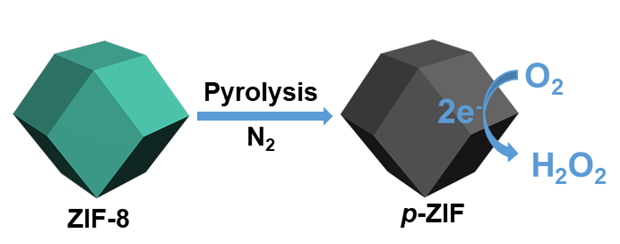
Electrochemical production of H2O2 from O2 via the two-electron reduction reaction (2e-ORR) is a green and safe process that is being heavily investigated. Zeolitic imidazolate frameworks (ZIF) as a subclass of metal-organic frameworks (MOFs) have attracted enormous scientific interests due to their high porosity, excellent mechanical stability, tunable surface properties, and exceptional chemical and thermal stabilities. Herein, a series of pyrolyzed ZIF catalysts (p-ZIF) were synthesized by treating 2-methylimidazole zinc salt (ZIF-8) at high temperatures (900, 950 and 1000 ℃). During pyrolysis, zinc was vaporized, leaving the metal-free nitrogen-doped porous graphitic carbon materials. The effects of the pyrolysis temperature on the structure and catalytic performance of the p-ZIF catalysts in 2e-ORR were systematically studied. In 2e-ORR in an acidic electrolyte, the p-ZIF catalysts displayed low overpotential, low Tafel slope, and negligible activity loss after 3000 cycles of accelerated durability testing (ADT). In particular, the p-ZIF-950 catalyst possessed the highest H2O2 partial current of 0.185 mA at 0 V vs. RHE, followed by the p-ZIF-900 (0.146 mA) and p-ZIF-1000 (0.135 mA) catalysts. The H2O2 selectivity over the p-ZIF-950 catalyst was also constantly higher than those over the other two p-ZIF catalysts across the potential range investigated and maximized at 89.2%. Highly efficient and durable H2O2 production was demonstrated on the p-ZIF-950 catalyst by the linear accumulation of H2O2 to 522 mmol•gcat-1 within 6 h, translating to an H2O2 production rate of 87 mmol•gcat-1•h-1. The morphology, composition, carbon defect, texture, and surface chemical state were characterized by techniques such as transmission electron microscopy (TEM), elemental analysis, Raman spectroscopy, N2 physisorption, and X-ray photoelectron spectroscopy (XPS). The p-ZIF catalysts not only nicely carried over the regular rhombic dodecahedral morphology of ZIF-8, but also possessed abundant amount of nitrogen, high specific surface area, and hierarchical pore structure. According to the characterization results, the specific surface area and carbon defect are unlikely the key factors that determine the catalytic performance, while the evolutions of the average pore size and surface content of graphitic N mimicked those of the H2O2 partial current and selectivity on the p-ZIF catalysts. In light of the literature works, the large pore size is proposed to facilitate the in-diffusion of O2 and out-diffusion of H2O2, while the graphitic N is able to enhance the activation of O2 and desorption of H2O2, both of which are beneficial to the kinetics of the 2e-ORR reaction.
Key words: hydrogen peroxide; ZIF-8; pyrolysis; electrocatalysis; oxygen reduction reaction
Dan Wang , Bo Feng , Xiaoxin Zhang , Yanan Liu , Yan Pei , Minghua Qiao , Baoning Zong . Nitrogen-doped Carbon Pyrolyzed from ZIF-8 for Electrocatalytic Oxygen Reduction to Hydrogen Peroxide[J]. Acta Chimica Sinica, 2022 , 80(6) : 772 -780 . DOI: 10.6023/A22010030
| [1] | Li, H. B.; Zheng, B.; Pan, Z. Y.; Zong, B. N.; Qiao, M. H. Front. Chem. Sci. Eng. 2018, 12, 124. |
| [2] | Siahrostami, S.; Verdaguer-Casadevall, A.; Karamad, M.; Deiana, D.; Malacrida, P.; Wickman, B.; Escudero-Escribano, M.; Paoli, E. A.; Frydendal, R.; Hansen, T. W.; Chorkendorff, I.; Stephens, I. E. L.; Rossmeisl, J. Nat. Mater. 2013, 12, 1137. |
| [3] | Campos-Martin, J. M.; Blanco-Brieva, G.; Fierro, J. L. G. Angew. Chem. Int. Ed. 2006, 45, 6962. |
| [4] | Yang, S.; Verdaguer-Casadevall, A.; Arnarson, L.; Silvioli, L.; Colic, V.; Frydendal, R.; Rossmeisl, J.; Chorkendorff, I.; Stephens, I. E. L. ACS Catal. 2018, 8, 4064. |
| [5] | Freakley, S. J.; He, Q.; Harrhy, J. H.; Lu, L.; Crole, D. A.; Morgan, D. J.; Ntainjua, E. N.; Edwards, J. K.; Carley, A. F.; Borisevich, A. Y.; Kiely, C. J.; Hutchings, G. J. Science 2016, 351, 965. |
| [6] | Sun, Y. Y.; Li, S.; Jovanov, Z. P.; Bernsmeier, D.; Wang, H.; Paul, B.; Wang, X.; Kühl, S.; Strasser, P. ChemSusChem 2018, 11, 3388. |
| [7] | Xia, C.; Xia, Y.; Zhu, P.; Fan, L.; Wang, H. T. Science 2019, 366, 226. |
| [8] | Zhang, J.; Zhang, H.; Cheng, M. J.; Lu, Q. Small 2020, 16, 1902845. |
| [9] | Yang, S.; Kim, J.; Tak, Y. J.; Soon, A.; Lee, H. Angew. Chem. Int. Ed. 2016, 55, 2058. |
| [10] | Wang, Y. L.; Li, S. S.; Yang, X. H.; Xu, G. Y.; Zhu, Z. C.; Chen, P.; Li, S. Q. J. Mater. Chem. A 2019, 7, 21329. |
| [11] | Zhao, H. Y.; Shen, X. Q.; Chen, Y.; Zhang, S. N.; Gao, P.; Zhen, X. J.; Li, X. H.; Zhao, G. H. Chem. Commun. 2019, 55, 6173. |
| [12] | Sa, Y. J.; Kim, J. H.; Joo, S. H. Angew. Chem. Int. Ed. 2019, 58, 1100. |
| [13] | Zhou, W.; Meng, X.; Gao, J.; Alshawabkeh, A. N. Chemosphere 2019, 225, 588. |
| [14] | Tian, X.; Zhao, X.; Su, Y. Q.; Wang, L.; Wang, H.; Dang, D.; Chi, B.; Liu, H.; Hensen, E. J. M.; Lou, X. W.; Xia, B. Y. Science 2019, 366, 850. |
| [15] | Gong, K.; Du, F.; Xia, Z.; Durstock, M.; Dai, L. Science 2009, 323, 760. |
| [16] | Sun, Y. Y.; Sinev, I.; Ju, W.; Bergmann, A.; Dresp, S.; Kühl, S.; Spöri, C.; Schmies, H.; Wang, H.; Bernsmeier, D.; Paul, B.; Schmack, R.; Kraehnert, R.; Roldan Cuenya, B.; Strasser, P. ACS Catal. 2018, 8, 2844. |
| [17] | Jiang, Y.; Ni, P.; Chen, C.; Lu, Y.; Yang, P.; Kong, B.; Fisher, A.; Wang, X. Adv. Energy Mater. 2018, 8, 1801909. |
| [18] | Melchionna, M.; Fornasiero, P.; Prato, M. Adv. Mater. 2019, 31, 1802920. |
| [19] | Lu, X. Q.; Cao, S. F.; Wei, X. F.; Li, S. R.; Wei, S. X. Acta Chim. Sinica 2020, 78, 1001. (in Chinese) |
| [19] | (鲁效庆, 曹守福, 魏晓飞, 李邵仁, 魏淑贤, 化学学报, 2020, 78, 1001.) |
| [20] | Jia, N.; Yang, T.; Shi, S.; Chen, X.; An, Z.; Chen, Y.; Yin, S.; Chen, P. ACS Sustainable Chem. Eng. 2020, 8, 2883. |
| [21] | Pizzutilo, E.; Kasian, O.; Choi, C. H.; Cherevko, S.; Hutchings, G. J.; Mayrhofer, K. J. J.; Freakley, S. J. Chem. Phys. Lett. 2017, 683, 436. |
| [22] | He, D. Q.; Zhong, L. J.; Gan, S. Y.; Xie, J. X.; Wang, W.; Liu, Z. B.; Guo, W.; Yang, X.; Niu, L. Electrochim. Acta 2021, 371, 137721. |
| [23] | Xi, J.; Yang, S.; Silvioli, L.; Cao, S.; Liu, P.; Chen, Q.; Zhao, Y.; Sun, H.; Hansen, J. N.; Haraldsted, J. B.; Kibsgaard, J.; Rossmeisl, J.; Bals, S.; Wang, S.; Chorkendorff, I. J. Catal. 2021, 393, 313. |
| [24] | Shen, R. G.; Chen, W. X.; Peng, Q.; Lu, S. Q.; Zheng, L. R.; Cao, X.; Wang, Y.; Zhu, W.; Zhang, J. T.; Zhuang, Z. B.; Chen, C.; Wang, D. G.; Li, Y. D. Chem 2019, 5, 2099. |
| [25] | Gao, J. J.; Yang, H. B.; Huang, X.; Hung, S. F.; Cai, W. Z.; Jia, C. M.; Miao, S.; Chen, H. M.; Yang, X. F.; Huang, Y. Q.; Zhang, T.; Liu, B. Chem 2020, 6, 658. |
| [26] | Jiang, K.; Back, S.; Akey, A. J.; Xia, C.; Hu, Y.F.; Liang, W. T.; Schaak, D.; Stavitski, E.; Norskov, J. K.; Siahrostami, S.; Wang, H. T. Nat. Commun. 2019, 10, 3997. |
| [27] | Sun, Y.; Silvioli, L.; Sahraie, N. R.; Ju, W.; Li, J.; Zitolo, A.; Li, S.; Bagger, A.; Arnarson, L.; Wang, X.; Moeller, T.; Bernsmeier, D.; Rossmeisl, J.; Jaouen, F.; Strasser, P. J. Am. Chem. Soc. 2019, 141, 12372. |
| [28] | Perazzolo, V.; Durante, C.; Pilot, R.; Paduano, A.; Zheng, J.; Rizzi, G. A.; Martucci, A.; Granozzi, G.; Gennaro, A. Carbon 2015, 95, 949. |
| [29] | Hasché, F.; Oezaslan, M.; Strasser, P.; Fellinger, T. J. Energy Chem. 2016, 25, 251. |
| [30] | Xia, Y.; Zhao, X. H.; Xia, C.; Wu, Z. Y.; Zhu, P.; Kim, J. Y.; Bai, X. W.; Gao, G. H.; Hu, Y. F.; Zhong, J.; Liu, Y. Y.; Wang, H.T. Nat. Commun. 2021, 12, 4225. |
| [31] | Chen, S. Y.; Luo, T.; Chen, K. J.; Lin, Y. Y.; Fu, J. W.; Liu, K.; Cai, C.; Wang, Q. Y.; Li, H. J. W.; Li, X. Q.; Hu, J. H.; Li, H. M.; Zhu, M. S.; Liu, M. Angew. Chem. Int. Ed. 2021, 60, 16607. |
| [32] | Xu, Z. H.; Shen, L. M.; Wu, Q.; Sun, T.; Xu, Y. Y.; Li, D. Q.; Du, L. Y.; Yang, L. J.; Wang, X. Z.; Hu, Z. Acta Chim. Sinica 2015, 73, 793. (in Chinese) |
| [32] | (许智慧, 沈丽明, 吴强, 孙涛, 徐宇洋, 黎聃勤, 杜玲玉, 杨立军, 王喜章, 胡征, 化学学报, 2015, 73, 793.) |
| [33] | Qin, M. C.; Fan, S. Y.; Wang, L.; Gan, G. Q.; Wang, X. Y.; Cheng, J.; Hao, Z. P.; Li, X. Y. J. Colloid Interface Sci. 2020, 562, 540. |
| [34] | Chen, S. C.; Chen, Z. H.; Siahrostami, S.; Higgins, D.; Nordlund, D.; Sokaras, D.; Kim, T. R.; Liu, Y. Z.; Yan, X. Z.; Nilsson, E.; Sinclair, R.; Nørskov, J. K.; Jaramillo, T. F.; Bao, Z. N. J. Am. Chem. Soc. 2018, 140, 7851. |
| [35] | Han, G. F.; Li, F.; Zou, W.; Karamad, M.; Jeon, J. P.; Kim, S. W.; Kim, S. J.; Bu, Y. F.; Fu, Z. P.; Lu, Y. L.; Siahrostami, S.; Baek, J. B. Nat. Commun. 2020, 11, 7293. |
| [36] | Zhao, K.; Su, Y.; Quan, X.; Liu, Y. M.; Chen, S.; Yu, H. T. J. Catal. 2018, 357, 118. |
| [37] | Dong, K.; Liang, J.; Wang, Y. Y.; Xu, Z. Q.; Liu, Q.; Luo, Y. L.; Li, T. S.; Li, L.; Shi, X. F.; Asiri, A. M.; Li, Q.; Ma, D. M.; Sun, X. P. Angew. Chem. Int. Ed. 2021, 60, 10583. |
| [38] | Fellinger, T.; Hasché, F.; Strasser, P.; Antonietti, M. J. Am. Chem. Soc. 2012, 134, 4072. |
| [39] | Zhou, Y.; Chen, G.; Zhang, J. J. J. Mater. Chem. A 2020, 8, 20849. |
| [40] | Zhang, L. J.; Su, Z. X.; Jiang, F. L.; Yang, L. L.; Qian, J. J.; Zhou, Y. F.; Li, W. M.; Hong, M. C. Nanoscale 2014, 6, 6590. |
| [41] | Guo, W. J.; Yu, J.; Dai, Z.; Hou, W. Z. Acta Chim. Sinica 2019, 77, 1203. (in Chinese) |
| [41] | (郭文娟, 于洁, 代昭, 侯伟钊, 化学学报, 2019, 77, 1203.) |
| [42] | Liu, Y.; Quan, X.; Fan, X.; Wang, H.; Chen, S. Angew. Chem. Int. Ed. 2015, 54, 6837. |
| [43] | Sun, Y. H.; Qi, Y. X.; Shen, Y.; Jing, C. J.; Chen, X. X.; Wang, X. X. Acta Chim. Sinica 2020, 78, 147. (in Chinese) |
| [43] | (孙延慧, 齐有啸, 申优, 井翠洁, 陈笑笑, 王新星, 化学学报, 2020, 78, 147.) |
| [44] | Kaneti, Y. V.; Dutta, S.; Hossain, M. S. A.; Shiddiky, M. J. A.; Tung, K. L.; Shieh, F. K.; Tsung, C. K.; Wu, K. C. W.; Yamauchi, Y. Adv. Mater. 2017, 29, 1700213. |
| [45] | Stanczyk, K.; Dziembaj, R.; Piwowarska, Z.; Witkowski, S. Carbon 1995, 33, 1383. |
| [46] | Amali, A. J.; Sun, J. K.; Xu, Q. Chem. Commun. 2014, 50, 1519. |
| [47] | Wei, S. J.; Li, A.; Liu, J. C.; Chen, W. X.; Gong, Y.; Zhang, Q. H.; Cheong, W. C.; Wang, Y.; Zheng, L. R.; Xiao, H.; Chen, C.; Wang, D. S.; Peng, Q.; Gu, L.; Han, X. D.; Li, J.; Li, Y. D. Nat. Nanotechnol. 2018, 13, 856. |
| [48] | Lu, Z.; Chen, G.; Siahrostami, S.; Chen, Z.; Liu, K.; Xie, J.; Liao, L.; Wu, T.; Lin, D.; Liu, Y.; Jaramillo, T. F.; Nørskov, J. K.; Cui, Y. Nat. Catal. 2018, 1, 156. |
| [49] | Perazzolo, V.; Daniel, G.; Brandiele, R.; Picelli, L.; Rizzi, G. A.; Isse, A. A.; Durante, C. Chem. Eur. J. 2021, 27, 1002. |
| [50] | Zhang, P.; Sun, F.; Xiang, Z. H.; Shen, Z. G.; Yun, J.; Cao, D. P. Energy Environ. Sci. 2014, 7, 442. |
| [51] | Gu, D. G.; Wang, F. F.; Yan, K.; Ma, R. G.; Wang, J. C. ACS Sustainable Chem. Eng. 2018, 6, 1951. |
| [52] | Li, L. Q.; Tang, C.; Zheng, Y.; Xia, B. Q.; Zhou, X. L.; Xu, H. L.; Qiao, S. Z. Adv. Energy Mater. 2020, 10, 2000789. |
| [53] | Chen, S. C.; Chen, Z. H.; Siahrostami, S.; Kim, T. R.; Nordlund, D.; Sokaras, D.; Nowak, S.; To, J. W. F.; Higgins, D.; Sinclair, R.; Nørskov, J. K.; Jaramillo, T. F.; Bao, Z. N. ACS Sustainable Chem. Eng. 2018, 6, 311. |
| [54] | Park, J.; Nabae, Y.; Hayakawa, T.; Kakimoto, M. ACS Catal. 2014, 4, 3749. |
| [55] | Iglesias, D.; Giuliani, A.; Melchionna, M.; Marchesan, S.; Criado, A.; Nasi, L.; Bevilacqua, M.; Tavagnacco, C.; Vizza, F.; Prato, M.; Fornasiero, P. Chem 2018, 4, 106. |
| [56] | Zhang, J. Y.; Zhang, G.; Jin, S. Y.; Zhou, Y. J.; Ji, Q. H.; Lan, H. C.; Liu, H. J.; Qu, J. H. Carbon 2020, 163, 154. |
| [57] | Contreras, E.; Dominguez, D.; Tiznado, H.; Guerrero-Sanchez, J.; Takeuchi, N.; Alonso-Nunez, G.; Contreras, O. E.; Oropeza- Guzman, M. T.; Romo-Herrera, J. M. Nanoscale 2019, 11, 2829. |
/
| 〈 |
|
〉 |