

Study on the Regulation of Alkali-earth Metal Ben (n=1~3) on the Structure of B12 Clusters
Received date: 2022-03-15
Online published: 2022-05-07
Supported by
Natural Science Foundation of Shanxi Province(201901D211283); School Fund of North China University(XJJ201917)
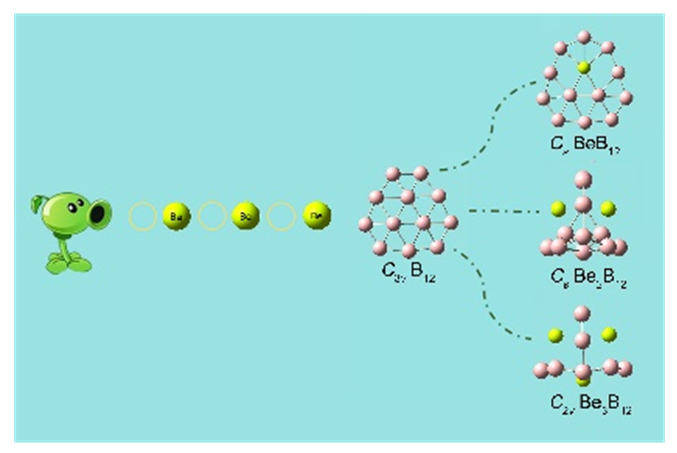
Based on first-principles, we systematically study the regulation of Ben (n=1~3) on the structure of B12 clusters. For all the low energy isomers of BenB12 (n=1~3) searched at the PBE/DZVP level, we further use PBE0 and TPSSh methods to calculate their relative energy and select the most optimal structure, and then calculate its single point energy at CCSD(T)/6-311+G(d). The results show that the global minimum structure of BeB12 is quasi-planar and has Cs symmetry at the CCSD(T)/6-311+G(d) level. However, the most stable structures of Be2B12 and Be3B12 are cage-like structures, and their symmetries are Cs and C2v, respectively. Subsequently, with the increase of the number of Be atoms, B12 has a dramatic transition from quasi-planar structure to cage-like structure, and Be atoms tend to be embedded in the B7 or B8 unit ring on the surface of the cage-like structure of B12, forming stable Be&B7 and Be&B8 units through ionic and covalent interaction to stabilize the cage-like structure. Furthermore, at 400 K, Cs BeB12 and Cs Be2B12 are kinetically stable, while some low-energy isomers of C2v Be3B12 can rheology each other. The natural bond orbital (NBO) analysis shows that the clusters Cs BeB12, Cs Be2B12, and C2v Be3B12 have obvious electron transfer, and the 2s orbital of Be atom loses electrons. And the Be—B bonds are mainly ionic, covalent bonds exist at the same time. Adaptive natural density partitioning (AdNDP) reveals the bonding pattern of BenB12 (n=1~3), and we find that both the σ bonds and the multicenter π bonds promote the stability of the whole molecule. The results indicate that the π bonds of Cs BeB12 conform to the Hückel (4n+2) rule and is aromatic, while the π bonds of Cs Be2B12, and C2v Be3B12 follow the spherical aromatic 2(n+1)2 (n=1) electron counting rule, with spherical aromaticity. In addition, we also predict the infrared and Raman spectra of the three structures in this text, which will provide theoretical basis for the experimentally synthesis and characterization of these structures in the future.
Hairu Li , Ceng Zhang , Sidian Li . Study on the Regulation of Alkali-earth Metal Ben (n=1~3) on the Structure of B12 Clusters[J]. Acta Chimica Sinica, 2022 , 80(7) : 888 -895 . DOI: 10.6023/A22030109
| [1] | Kroto, H. W.; Heath, J. R.; O'Brien, S. C.; Curl, R. F.; Smalley, R. E. Nature 1985, 318, 162. |
| [2] | Krätschmer, W.; Lamb, L. D.; Fostiropoulos, K.; Huffman, D. R. Nature 1990, 347, 354. |
| [3] | Iijima, S. Nature 1991, 354, 56. |
| [4] | Iijima, S.; Ichihashi, T. Nature 1993, 363, 603. |
| [5] | Alexandrova, A. N.; Boldyrev, A. I.; Zhai, H. J.; Wang, L. S. Coordin. Chem. Rev. 2006, 250, 2811. |
| [6] | Sergeeva, A. P.; Popov, I. A.; Piazza, Z. A.; Li, W. L.; Romanescu, C.; Wang, L. S.; Boldyrev, Alexander I. Acc. Chem. Res. 2014, 47, 1349. |
| [7] | Wang, L. S. Int. Rev. Phys. Chem. 2016, 35, 69. |
| [8] | Zhai, H. J.; Zhao, Y. F.; Li, W. L.; Chen, Q.; Bai, H.; Hu, H. S.; Piazza, Z. A.; Tian, W. J.; Lu, H. G.; Wu, Y. B.; Mu, Y. W.; Wei, G. F.; Liu, Z. P.; Li, J.; Li, S. D.; Wang, L. S. Nat. Chem. 2014, 6, 727. |
| [9] | Chen, Q.; Li, H. R.; Tian, W. J.; Lu, H. G.; Zhai, H. J.; Li, S. D. Phys. Chem. Chem. Phys. 2016, 18, 14186. |
| [10] | Romanescu, C.; Galeev, T. R.; Li, W. L.; Boldyrev, A. I.; Wang, L. S. Angew. Chem., Int. Ed. 2011, 50, 9334. |
| [11] | Li, W. L.; Romanescu, C.; Galeev, T. R.; Piazza, Z. A.; Boldyrev, A. I.; Wang, L. S. J. Am. Chem. Soc. 2012, 134, 165-168. |
| [12] | Galeev, T. R.; Romanescu, C.; Li, W. L.; Wang, L. S.; Boldyrev, A. I. Angew. Chem., Int. Ed. 2012, 51, 2101. |
| [13] | Romanescu, C.; Galeev, T. R.; Li, W. L.; Boldyrev, A. I.; Wang, L. S. J. Chem. Phys. 2013, 138, 134315. |
| [14] | Li, W. L.; Ivanov, A. S.; Federi, J.; Romanescu, C.; ernuša?k, I.; Boldyrev, A. I.; Wang, L. S. J. Chem. Phys. 2013, 139, 104312. |
| [15] | Romanescu, C.; Galeev, T. R.; Li, W. L.; Boldyrev, A. I.; Wang, L. S. Acc. Chem. Res. 2013, 46, 350. |
| [16] | Popov, I. A.; Li, W. L.; Piazza, Z. A.; Boldyrev, A. I.; Wang, L. S. J. Phys. Chem. A 2014, 118, 8098. |
| [17] | Popov, I. A.; Jian, T.; Lopez, G. V.; Boldyrev, A. I.; Wang, L. S. Nat. Commun. 2015, 6, 8654. |
| [18] | Jian, T.; Li, W. L.; Popov, I. A.; Lopez, G. V.; Chen, X.; Boldyrev, A. I.; Li, J.; Wang, L. S. J. Chem. Phys. 2016, 144, 154310. |
| [19] | Jian, T.; Li, W. L.; Chen, X.; Chen, T. T.; Lopez, G. V.; Li, J.; Wang, L. S. Chem. Sci. 2016, 7, 7020. |
| [20] | Li, W. L.; Jian, T.; Chen, X.; Chen, T. T.; Lopez, G. V.; Li, J.; Wang, L. S. Angew. Chem., Int. Ed. 2016, 55, 7358. |
| [21] | Li, H. R.; Liu, H.; Tian, X. X.; Zan, W. Y.; Mu, Y. W.; Lu, H. G.; Li, J.; Wang, Y. K.; Li, S. D. Phys. Chem. Chem. Phys. 2017, 19, 27025. |
| [22] | Li, H. R.; Liu, H.; Lu, X. Q.; Zan, W. Y.; Tian, X. X.; Lu, H. G.; Wu, Y. B.; Mu, Y. W.; Li, S. D. Nanoscale 2018, 10, 7451. |
| [23] | Li, H. R. Ph.D. Dissertation, Shanxi University, Taiyuan, 2019. (in Chinese) |
| [23] | (李海茹, 博士论文, 山西大学, 太原, 2019.) |
| [24] | Wang, Y. J.; Feng, L. Y.; Guo, J. C.; Zhai, H. J. Chem-Asian J. 2017, 12, 2899. |
| [25] | Li, S. X.; Chen, D. L.; Zhang, Z. L.; Long, Z. W. Acta Phys. Sin. 2020, 69, 193101. (in Chinese) |
| [25] | (李世雄, 陈德良, 张正良, 隆正文, 物理学报, 2020, 69, 193101.) |
| [26] | Dong, X.; Jalife, S.; Vásquez-Espinal, A.; Ravell, E.; Pan, S.; Cabellos, J. L.; Liang, W. Y.; Cui, Z. H.; Merino, G. Angew. Chem. 2018, 130, 1. |
| [27] | Feng, L. Y. Ph.D. Dissertation, Shanxi University, Taiyuan, 2021. (in Chinese) |
| [27] | (冯林雁, 博士论文, 山西大学, 太原, 2021.) |
| [28] | Cui, Z. H.; Yang, W. S.; Zhao, L. L.; Ding, Y. H.; Frenking, G. Angew. Chem., Int. Ed., 2016, 55, 7841. |
| [29] | Feng, L. Y.; Guo, J. C.; Li, P. F.; Zhai, H. J. Phys. Chem. Chem Phys. 2018, 20, 22719. |
| [30] | Guo, J. C.; Feng, L. Y.; Wang, Y. J.; Jalife, S.; Vásquez-Espinal, A.; Cabellos, J. L.; Pan, S.; Merino, G.; Zhai, H. J. Angew. Chem., Int. Ed. 2017, 56, 10174. |
| [31] | Feng, L. Y.; Wang, K.; Zhai, H. J. Phys. Chem. Chem. Phys. 2020, 22, 25574. |
| [32] | Feng, L. Y.; Guo, J. C.; Li, P. F.; Zhai, H. J. Chem. Asian J. 2020, 15, 1094. |
| [33] | Zhai, H. J.; Kiran, B.; Li, J.; Wang, L. S. Nat. Mater. 2003, 2, 827. |
| [34] | Zhao, Y. F.; Chen, X.; Li, J. Nano Research. 2017, 10, 3407. |
| [35] | Chen, X.; Zhao, Y. F.; Wang, L. S.; Li, J. Comput. Theor. Chem. 2017, 1107, 57. |
| [36] | Adamo, C.; Barone, V. J. Chem. Phys. 1999, 110, 6158. |
| [37] | Tao, J.; Perdew, J. P.; Staroverov, V. N.; Scuseria, G. E. Phys. Rev. Lett. 2003, 91, 146401. |
| [38] | Krishnan, R.; Binkley, J. S.; Seeger, R.; Pople, J. A. J. Chem. Phys. 1980. 72, 650. |
| [39] | Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Petersson, G. A.; Nakatsuji, H.; Li, X.; Caricato, M.; Marenich, A. V.; Bloino, J.; Janesko, B. G.; Gomperts, R.; Mennucci, B.; Hratchian, H. P.; Ortiz, J. V.; Izmaylov, A. F.; Sonnenberg, J. L.; Williams-Young, D.; Ding, F.; Lipparini, F.; Egidi, F.; Goings, J.; Peng, B.; Petrone, A.; Henderson, T.; Ranasinghe, D.; Zakrzewski, V. G.; Gao, J.; Rega, N.; Zheng, G.; Liang, W.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Throssell, K.; Montgomery Jr., J. A.; Peralta, J. E.; Ogliaro, F.; Bearpark, M. J.; Heyd, J. J.; Brothers, E. N.; Kudin, K. N.; Staroverov, V. N.; Keith, T. A.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A. P.; Burant, J. C.; Iyengar, S. S.; Tomasi, J.; Cossi, M.; Millam, J. M.; Klene, M.; Adamo, C.; Cammi, R.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Farkas, O.; Foresman, J. B.; Fox, D. J. Gaussian 16 Revision, A. 03, Gaussian Inc., Wallingford, CT, 2016. |
| [40] | Zubarev, D. Y.; Boldyrev, A. I. Phys. Chem. Chem. Phys. 2008, 10, 5207. |
| [41] | Glendening, E. D.; Badenhoop, J. K.; Reed, A. E.; Carpenter, J. E.; Bohmann, J. A.; Morales, C. M.; Weinhold, F.NBO 5.0/6.0, Theoretical Chemistry Institute, University of Wisconsin, Madison, 2001. |
| [42] | Vondele, J. V.; Krack, M.; Mohamed, F.; Parrinello, M.; Chassaing, T.; Hutter, J. Comput. Phys. Commun. 2005, 167, 103. |
| [43] | Pyykkö, P. J. Phys. Chem. A. 2015, 119, 2326. |
| [44] | Li, S. D.; Zhai, H. J.; Wang, L. S. J. Am. Chem. Soc. 2008, 130, 2573. |
| [45] | Kalemos, A. J. Chem. Phys. 2016, 145, 214302. |
| [46] | Wang, G.; Zhou, M.; Goettel, J. T.; Schrobilgen, G. J.; Su, J.; Li, J.; Schlöder, T.; Riedel, S. Nature 2014, 514, 475. |
/
| 〈 |
|
〉 |