

Theoretical Study on the Multiple Resonance Thermally Activated Delayed Fluorescence Process
Received date: 2022-11-24
Online published: 2023-01-11
Supported by
National Natural Science Foundation of China for the General Program(21873036)
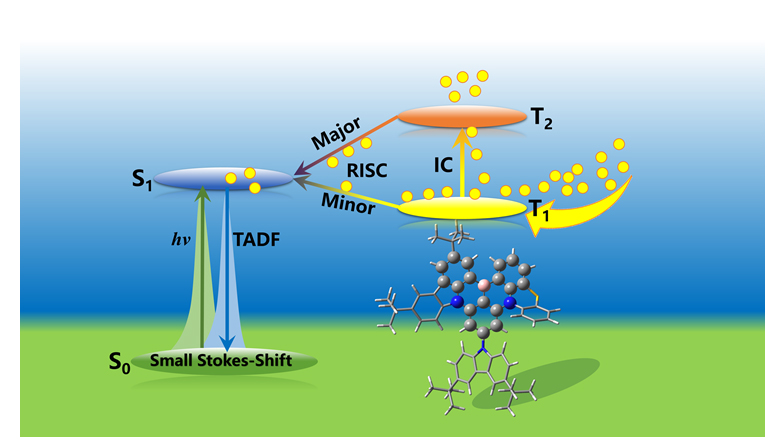
In recent years, the multiple resonance thermally activated delayed fluorescence (MR-TADF) has become a hot topic due to the high fluorescence quantum yield and small Stokes shift. In traditional TADF molecules, the lowest triplet excited state (T1) can convert into the lowest singlet excited state (S1) through the reverse intersystem crossing (RISC), and then return to the ground state (S0) by the radiative fluorescence emission. However, recent studies found that in MR-TADF molecules, the higher triplet excited state (T2) might play an important role. Thus, the detailed mechanism for the luminescence in MR-TADF molecules, especially for the effect of higher triplet excited state, has still been an indefinite problem. In this work, the luminescence mechanism of four newly reported MR-TADF molecules (BCz-BN, 2PTZ-BN, CZ-PTZ-BN and 2Cz-PTZ-BN) which are substituted by tert-butyl-carbazole and phenothiazine groups has been systematically investigated by the first-principle calculation and kinetic time evolution. The results showed that the phenothiazine group can significantly increase the spin-orbit coupling (SOC) and increase the fluorescence quantum yield. More importantly, by computing the rate constants for the TADF process, we found that the internal conversion (IC) rate between two triplet excited states (T1 and T2) is much faster than the radiative and non-radiative rates, and meanwhile the rate of the reverse intersystem crossing of T2→S1 is also faster than the one of T1→S1. Therefore, the TADF process of the above molecules should follow the T1→T2→S1→S0 process. The above findings were also confirmed by the further kinetic time evolution. In the early stage, the system was mainly dominated by the internal conversion between T1 and T2. With the time evolution, the energy gradually transferred from T2 to S1, and finally emitted fluorescence on S1. However, if T2 is not involved in the time evolution, the TADF process will be significantly slowed down, which could further confirm the importance of T2 in the TADF process. This work reveals the nature of the TADF process which could be of great importance for designing and synthesizing new TADF molecules.
Shaoqin Zhang , Meiqing Li , Zhongjun Zhou , Zexing Qu . Theoretical Study on the Multiple Resonance Thermally Activated Delayed Fluorescence Process[J]. Acta Chimica Sinica, 2023 , 81(2) : 124 -130 . DOI: 10.6023/A22110472
| [1] | (a) Uoyama, H.; Goushi, K.; Shizu, K.; Nomura, H.; Adachi, C. Nature. 2012, 492, 234. |
| [1] | (b) Adachi, C. Jpn. J. Appl. Phys. 2014, 53, 060101. |
| [2] | (a) Liu, Y.; Li, C.; Ren, Z.; Yan, S.; Bryce, M. R. Nat. Rev. Mater. 2018, 3, 18020. |
| [2] | (b) Chen, D.; Li, W.; Gan, L.; Wang, Z.; Li, M.; Su, S.-J. Mater. Sci. Eng. R Rep. 2020, 142, 100581. |
| [2] | (c) Xiao, Y.; Wang, H.; Xie, Z.; Shen, M.; Huang, R.; Miao, Y.; Liu, G.; Yu, T.; Huang, W. Chem. Sci. 2022, 13, 8906. |
| [3] | (a) Dias, F. B.; Penfold, T. J.; Monkman, A. P. Methods Appl. Fluoresc. 2017, 5, 012001. |
| [3] | (b) Yang, Z.; Mao, Z.; Xie, Z.; Zhang, Y.; Liu, S.; Zhao, J.; Xu, J.; Chi, Z.; Aldred, M. P. Chem. Soc. Rev. 2017, 46, 915. |
| [4] | (a) Cao, H.; Hou, P. Acta Chim. Sinica. 2022, 80, 1476. (in Chinese) |
| [4] | (曹洪涛, 侯鹏飞, 化学学报, 2022, 80, 1476.) |
| [4] | (b) Wang, T.; Hua, X.; Yu, Y.; Yuan, Y.; Feng, M.; Jiang, Z. Chin. J. Org. Chem. 2019, 39, 1436. (in Chinese) |
| [4] | (王彤彤, 华晓晨, 郁友军, 袁熠, 冯敏强, 蒋佐权, 有机化学, 2019, 39, 1436.) |
| [4] | (c) Zheng, Y.; Xie, Q.; Wang, B. Chin. J. Org. Chem. 2016, 36, 803. (in Chinese) |
| [4] | (郑月游, 谢琼琳, 王炳喜, 有机化学, 2016, 36, 803.) |
| [5] | (a) Ikeda, N.; Oda, S.; Matsumoto, R.; Yoshioka, M.; Fukushima, D.; Yoshiura, K.; Yasuda, N.; Hatakeyama, T. Adv. Mater. 2020, 32, 2004072. |
| [5] | (b) Kondo, Y.; Yoshiura, K.; Kitera, S.; Nishi, H.; Oda, S.; Gotoh, H.; Sasada, Y.; Yanai, M.; Hatakeyama, T. Nat. Photonics. 2019, 13, 678. |
| [6] | Hatakeyama, T.; Shiren, K.; Nakajima, K.; Nomura, S.; Nakatsuka, S.; Kinoshita, K.; Ni, J.; Ono, Y.; Ikuta, T. Adv. Mater. 2016, 28, 2777. |
| [7] | (a) Lee, H.; Braveenth, R.; Park, J. D.; Jeon, C. Y.; Lee, H. S.; Kwon, J. H. ACS Appl. Mater. Interfaces. 2022, 14, 36927. |
| [7] | (b) Kim, H. J.; Yasuda, T. Adv. Opt. Mater. 2022, 1, 1765. |
| [8] | (a) Madayanad Suresh, S.; Hall, D.; Beljonne, D.; Olivier, Y.; Zysman‐Colman, E. Adv. Funct. Mater. 2020, 30, 1908677. |
| [8] | (b) Nakanotani, H.; Tsuchiya, Y.; Adachi, C. Chem. Lett. 2021, 50, 938. |
| [9] | (a) Zhang, Y.; Zhang, D.; Wei, J.; Liu, Z.; Lu, Y.; Duan, L. Angew. Chem. Int. Ed. 2019, 58, 16912. |
| [9] | (b) Xu, Y.; Wang, Q.; Cai, X.; Li, C.; Wang, Y. Adv. Mater. 2021, 33, 2100652. |
| [9] | (c) Qi, Y.; Ning, W.; Zou, Y.; Cao, X.; Gong, S.; Yang, C. Adv. Funct. Mater. 2021, 31, 2102017. |
| [9] | (d) Yang, M.; Park, I. S.; Yasuda, T. J. Am. Chem. Soc. 2020, 142, 19468. |
| [9] | (e) Yang, M.; Shikita, S.; Min, H.; Park, I. S.; Shibata, H.; Amanokura, N.; Yasuda, T. Angew. Chem. Int. Ed. 2021, 60, 23142. |
| [10] | Northey, T.; Penfold, T. J. Org. Electron. 2018, 59, 45. |
| [11] | (a) Kim, J. U.; Park, I. S.; Chan, C.-Y.; Tanaka, M.; Tsuchiya, Y.; Nakanotani, H.; Adachi, C. Nat. Commun. 2020, 11, 1765. |
| [11] | (b) Hua, T.; Zhan, L.; Li, N.; Huang, Z.; Cao, X.; Xiao, Z.; Gong, S.; Zhou, C.; Zhong, C.; Yang, C. Chem. Eng. J. 2021, 426, 131169. |
| [11] | (c) Luo, X.; Ni, H.; Ma, H.; Qu, Z.; Wang, J.; Zheng, Y.; Zuo, J. Adv. Opt. Mater. 2022, 10, 2102513. |
| [12] | Shizu, K.; Kaji, H. Commun. Chem. 2022, 5, 53. |
| [13] | Liu, F.; Cheng, Z.; Jiang, Y.; Gao, L.; Liu, H.; Liu, H.; Feng, Z.; Lu, P.; Yang, W. Angew. Chem. Int. Ed. 2022, 61, e202116927. |
| [14] | Yanai, T.; Tew, D. P.; Handy, N. C. Chem. Phys. Lett. 2004, 393, 51. |
| [15] | (a) Freundorfer, K.; Kats, D.; Korona, T.; Schütz, M. J. Chem. Phys. 2010, 133, 244110. |
| [15] | (b) Kats, D.; Schütz, M. J. Chem. Phys. 2009, 131, 124117. |
| [15] | (c) Kats, D.; Schütz, M. Z. Phys. Chem. 2010, 224, 601. |
| [16] | Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Petersson, G. A.; Nakatsuji, H.; Li, X.; Caricato, M.; Marenich, A. V.; Bloino, J.; Janesko, B. G.; Gomperts, R.; Mennucci, B.; Hratchian, H. P.; Ortiz, J. V.; Izmaylov, A. F.; Sonnenberg, J. L.; Williams-Young, D.; Ding, F.; Lipparini, F.; Egidi, F.; Goings, J.; Peng, B.; Petrone, A.; Henderson, T.; Ranasinghe, D.; Zakrzewski, V. G.; Gao, J.; Rega, N.; Zheng, G.; Liang, W.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Throssell, K.; Montgomery, J. A., Jr.; Peralta, J. E.; Ogliaro, F.; Bearpark, M. J.; Heyd, J. J.; Brothers, E. N.; Kudin, K. N.; Staroverov, V. N.; Keith, T. A.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A. P.; Burant, J. C.; Iyengar, S. S.; Tomasi, J.; Cossi, M.; Millam, J. M.; Klene, M.; Adamo, C.; Cammi, R.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Farkas, O.; Foresman, J. B.; Fox, D. J. Gaussian 16, Revision A.03, Gaussian, Inc., Wallingford CT, 2016. |
| [17] | (a) Werner, H.-J.; Knowles, P. J.; Knizia, G.; Manby, F. R.; Schütz, M. WIREs Comput. Mol. Sci. 2012, 2, 242. |
| [17] | (b) Werner, H.-J.; Knowles, P. J.; Manby, F. R.; Black, J. A.; Doll, K.; He?elmann, A.; Kats, D.; K?hn, A.; Korona, T.; Kreplin, D. A.; Ma, Q.; Miller, T. F.; Mitrushchenkov, A.; Peterson, K. A.; Polyak, I.; Rauhut, G.; Sibaev, M. J. Chem. Phys. 2020, 152, 144107. |
| [18] | Kállay, M.; Nagy, P. R.; Mester, D.; Rolik, Z.; Samu, G.; Csontos, J.; Csóka, J.; Szabó, P. B.; Gyevi-Nagy, L.; Hégely, B.; Ladjánszki, I.; Szegedy, L.; Ladóczki, B.; Petrov, K.; Farkas, M.; Mezei, P. D.; Ganyecz, á. J. Chem. Phys. 2020, 152, 074107. |
| [19] | (a) Shuai, Z. Chin. J. Chem. 2020, 38, 1223. |
| [19] | (b) Shuai, Z.; Peng, Q. Natl. Sci. Rev. 2017, 4, 224. |
| [19] | (c) Shuai, Z.; Peng, Q. Phys. Rep. 2014, 537, 123. |
| [20] | Neese, F. WIREs Comput. Mol. Sci. 2022, 12, e1606. |
| [21] | Becke, A. D. J. Chem. Phys. 1993, 98, 5648. |
| [22] | Chai, J.-D.; Head-Gordon, M. Phys. Chem. Chem. Phys. 2008, 10, 6615. |
| [23] | Zhao, Y.; Truhlar, D. G. Theor. Chem. Acc. 2008, 120, 215. |
/
| 〈 |
|
〉 |