

Progress on the Transition Metal-catalyzed Cross-coupling Reaction of Thioesters
Received date: 2023-01-17
Online published: 2023-03-03
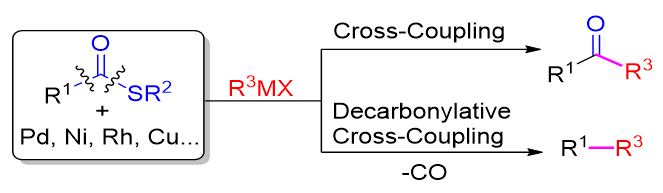
Thioesters with intriguing chemistry and reactivity are widely used in pharmaceuticals, pesticides, perfume & flavors and materials. Thioesters are versatile building blocks in organic synthesis and are also often employed as electrophilic acylating reagents in substitution reactions, such as Corey-Nicolaou macrolactonizations and native chemical ligation for peptide connection. Thioesters can be easily obtained from direct thioesterification of carboxylic acids with various thiol sources. Whereas more efforts have been made to develop new reactions employing thioesters, their application in organic synthesis is rare compared with other carboxylic acid derivatives. Transition-metal-catalyzed C—S bond activation paves a new avenue to exploit thioester. Many new transformations have been developed, including reduction to aldehydes, decarbonylation to thioethers, addition to unsaturated bonds, and cross-coupling of thioesters with organometallic reagents. Compared with other carboxylic acid derivatives, thioesters are stable but reactive and could undergo the oxidative addition of C(O)—S to low-valent transition-metal species, generating the C(O)—M—S. Transition-metal-catalyzed cross-coupling reaction of thioesters have been extensively studied in the last two decades, and providing alternative and efficient ways to construct C—C bonds. Despite the reaction between thioester and selected organometallic reagents to furnish ketones, thioesters could also be employed as decarbonylative coupling electrophiles. The advances in transition-metal-catalyzed cross-coupling reaction of thioesters are summarized in this review, which is presented by the categories of transition metals including Pd, Ni, Cu and Rh. Simultaneously, synthetic applications of transition-metal-catalyzed cross-coupling reaction of thioesters in nature products and pharmaceuticals are discussed. Attention is also paid to the asymmetric cross-coupling reaction of thioesters reported in recent years, the application of cross-coupling reaction of thioesters in tandem reactions and functional group transformations.
Mingliang Han , Lihua Xu . Progress on the Transition Metal-catalyzed Cross-coupling Reaction of Thioesters[J]. Acta Chimica Sinica, 2023 , 81(4) : 381 -392 . DOI: 10.6023/A23010013
| [1] | Pietrocola, F.; Galluzzi, L.; Bravo-San Pedro, J. M.; Madeo, F.; Kroemer, G. Cell Metab. 2015, 21, 8051. |
| [2] | (a) Wang, N. Z.; Saidhareddy, P.; Jiang, X. F. Nat. Prod. Rep. 2020, 37, 246. |
| [2] | (b) Adamczyk, M.; Fishpaugh, J. R. Tetrahedron Lett. 1996, 37, 4305. |
| [2] | (c) Wang, M.; Wang, C. H.; Jiang, X. F. Chin. J. Org. Chem. 2019, 39, 2139. (in Chinese) |
| [2] | (王明, 王翠红, 姜雪峰, 有机化学, 2019, 39, 2139). |
| [2] | (d) Ye, Q.; Cao, W. G.; Gao, J. S. Chin. J. Org. Chem. 2001, 21, 697. (in Chinese) |
| [2] | (叶青, 曹卫国, 高金森, 有机化学, 2001, 21, 697.) |
| [2] | (e) Aksakal, S.; Aksakal, R.; Becer, C. R. Polym. Chem. 2018, 9, 4507. |
| [3] | (a) Huxtable, R. J. Biochemistry of Sulfur, Springer, Boston, 1986, p. 220. |
| [3] | (b) Wang, X. C.; Ji, Z. Y.; Liu, J.; Wang, B. F.; Jin, H.; Zhang, L. X. Acta Chim. Sinica 2023, 81, 64. (in Chinese) |
| [3] | (王晓晨, 季泽尧, 刘健, 王炳福, 金辉, 张立新, 化学学报, 2023, 81, 64). |
| [4] | Staunton, J.; Weissman, K. J. Nat. Prod. Rep. 2001, 18, 380. |
| [5] | (a) Fukuyama, T.; Tokuyama, H. Aldrichimica Acta 2004, 37, 87. |
| [5] | (b) Fukuyama, T.; Lin, S. C.; Li, L. J. Am. Chem. Soc. 1990, 112, 7050. |
| [5] | (c) Tokuyama, H.; Yokoshima, S.; Lin, S. C.; Li, L.; Fukuyama, T. Synthesis 2002, 8, 1121. |
| [5] | (d) Kimura, M.; Seki, M. Tetrahedron Lett. 2004, 45, 3219. |
| [5] | (e) Asadi, M.; Bonke, S.; Polyzos, A.; Lupton, D. W. ACS Catal. 2014, 4, 2070. |
| [6] | (a) Osakada, K.; Yamamoto, T.; Yamamoto, A. Tetrahedron Lett. 1987, 28, 6321. |
| [6] | (b) Kato, T.; Kuniyasu, H.; Kajiura, T.; Minami, Y.; Ohtaka, A.; Kinomoto, M.; Terao, J.; Kurosawa, H.; Kambe, N. Chem. Commun. 2006, 868. |
| [6] | (c) Ishitobi, K.; Isshiki, R.; Asahara, K. K.; Lim, C.; Muto, K.; Yamaguchi, J. Chem. Lett. 2018, 47, 756. |
| [6] | (d) Ichiishi, N.; Malapit, C. A.; Wozniak, ?.; Sanford, M. S. Org. Lett. 2018, 20, 44. |
| [6] | (e) Lee, S. C.; Liao, H. H.; Chatupheeraphat, A.; Rueping, M. Chem. Eur. J. 2018, 24, 3608. |
| [6] | (f) Liu, C. W.; Szostak, M. Chem. Commun. 2018, 54, 2130. |
| [7] | (a) Hua, R.; Takeda, H.; Onozawa, S.; Abe, Y.; Tanaka, M. J. Am. Chem. Soc. 2001, 123, 2899. |
| [7] | (b) Sugoh, K.; Kuniyasu, H.; Sugae, T.; Ohtaka, A.; Takai, Y.; Tanaka, A.; Machino, C.; Kambe, N.; Kurosawa, H. J. Am. Chem. Soc. 2001, 123, 5108. |
| [7] | (c) Toyofuku, M.; Fujiwara, S.; Shin-Ike, T.; Kuniyasu, H.; Kambe, N. J. Am. Chem. Soc. 2005, 127, 9706. |
| [7] | (d) Minami, Y.; Kuniyasu, H.; Miyafuji, K.; Kambe, N. Chem. Commun. 2009, 3080. |
| [7] | (e) Arisawa, M.; Tanii, S.; Yamada, T.; Yamaguchi, M. Tetrahedron 2015, 71, 6449. |
| [7] | (f) Inami, T.; Kurahashi, T.; Matsubara, S. Chem. Commun. 2015, 51, 1285. |
| [8] | (a) Prokopcová, H.; Kappe, C. O. Angew. Chem. Int. Ed. 2009, 48, 2276. |
| [8] | (b) Cheng, H.-G.; Chen, H.; Liu, Y.; Zhou, Q. H. Asian J. Org. Chem. 2018, 7, 490. |
| [8] | (c) Hirschbeck, V.; Gehrtz, P. H.; Fleischer, I. Chem. Eur. J. 2018, 24, 7092. |
| [9] | (a) Yin, L. X.; Liebscher, J. Chem. Rev. 2007, 107, 133. |
| [9] | (b) Transition Metals for Organic Synthesis, 2nd ed., Eds.: Beller, M.; Bolm, C., Wiley-VCH, Weinheim, 2004. |
| [10] | (a) Lou, J.; Wang, Q. N.; Wu, P.; Wang, H. M.; Zhou, Y.-G.; Yu, Z. K. Chem. Soc. Rev. 2020, 49, 4307. |
| [10] | (b) Wang, L. D.; He, W.; Yu, Z. K. Chem. Soc. Rev. 2013, 42, 599. |
| [11] | Tokuyama, H.; Yokoshima, S.; Yamashita, T.; Fukuyama, T. Tetrahedron Lett. 1998, 39, 3189. |
| [12] | Liebeskind, L. S.; Srogl, J. J. Am. Chem. Soc. 2000, 122, 11260. |
| [13] | Chernyshev, V. M.; Ananikov, V. P. ACS Catal. 2022, 12, 1180. |
| [14] | Hayashi, Y.; Itoh, T.; Fukuyama, T. Org. Lett. 2003, 5, 2235. |
| [15] | Ueda, H.; Satoh, H.; Matsumoto, K.; Sugimoto, K.; Fukuyama, T.; Tokuyama, H. Angew. Chem. Int. Ed. 2009, 48, 7600. |
| [16] | Lee, J. H.; Kishi, Y. J. Am. Chem. Soc. 2016, 138, 7178. |
| [17] | Hall, D. G. Boronic Acids, Wiley-VCH, Weinheim, 2005. |
| [18] | Yang, S. Y.; Yu, X.; Szostak M. ACS Catal. 2023, 13, 1848. |
| [19] | Yu, Y.; Liebeskind, L. S. J. Org. Chem. 2004, 69, 3554. |
| [20] | (a) Ridgway, B. H.; Woerpel, K. A. J. Org. Chem. 1998, 63, 458. |
| [20] | (b) Rivera, I.; Colberg, J. C.; Soderquist, J. A. Tetrahedron Lett. 1992, 33, 6919. |
| [20] | (c) Netherton, M. R.; Dai, C.; Neuschuetz, K.; Fu, G. C. J. Am. Chem. Soc. 2001, 123, 10099. |
| [21] | Tsuna, K.; Noguchi, N.; Nakada, M. Tetrahedron Lett. 2011, 52, 7202. |
| [22] | Yang, H.; Li, H.; Wittenberg, R.; Egi, M.; Huang, W.; Liebeskind, L. S. J. Am. Chem. Soc. 2007, 129, 1132. |
| [23] | Yang, H.; Liebeskind, L. S. Org. Lett. 2007, 9, 2993. |
| [24] | Dandepally, S. R.; Williams, A. L. Tetrahedron Lett. 2010, 51, 5753. |
| [25] | Vasiljevik, T.; Groer, C. E.; Lehner, K.; Navarro, H.; Prisinzano, T. E. J. Nat. Prod. 2014, 77, 1817. |
| [26] | Cao, Y. F.; Li, L. J.; Liu, M.; Xu, H.; Dai, H. X. J. Org. Chem. 2020, 85, 4475. |
| [27] | (a) O’Hagan, D. Chem. Soc. Rev. 2008, 37, 308. |
| [27] | (b) Purser, S.; Moore, P. R.; Swallow, S.; Gouverneur, V. Chem. Soc. Rev. 2008, 37, 320. |
| [27] | (c) Kirk, K. L. Org. Process Res. Dev. 2008, 12, 305. |
| [28] | Yi, X.; Cao, Y. F.; Wang, X.; Xu, H.; Ban, S. R.; Dai, H. X. Tetrahedron Lett. 2020, 61, 151780. |
| [29] | Wang, M.; Dai, Z. H.; Jiang, X. F. Nat. Commun. 2019, 10, 2661. |
| [30] | Wittenberg, R.; Srogl, J.; Egi, M.; Liebeskind, L. S. Org. Lett. 2003, 5, 3033. |
| [31] | Saito, T.; Fuwa, H.; Sasaki, M. Org. Lett. 2009, 11, 5274. |
| [32] | Fenneteau, J.; Vallerotto, S.; Ferrié, L.; Figadère, B. Tetrahedron Lett. 2015, 56, 3758. |
| [33] | Ferrié, L.; Fenneteau, J.; Figadère, B. Org. Lett. 2018, 20, 3192. |
| [34] | (a) Morita, A.; Kuwahara, S. Org. Lett. 2006, 8, 1613. |
| [34] | (b) Morita, A.; Kiyota, H.; Kuwahara, S. Biosci. Biotechnol. Biochem. 2006, 70, 2564. |
| [35] | Kang, G.; Han, S. J. Am. Chem. Soc. 2022, 144, 8932. |
| [36] | Pearson, R. G. Chemical Hardness, Wiley-VCH, Weinheim, 1997. |
| [37] | Fausett, B. W.; Liebeskind, L. S. J. Org. Chem. 2005, 70, 4851. |
| [38] | Mehta, V. P.; Sharma, A.; Van der Eycken, E. Adv. Synth. Catal. 2008, 350, 2174. |
| [39] | Srogl, J.; Allred, G. D.; Liebeskind, L. S. J. Am. Chem. Soc. 1997, 119, 12376. |
| [40] | Savarin, C.; Srogl, J.; Liebeskind, L. S. Org. Lett. 2000, 2, 3229. |
| [41] | Thottumkara, A. P.; Kurokawa, T.; Du Bois, J. Chem. Sci. 2013, 4, 2686. |
| [42] | Liu, M.; Liu, Y. W.; Xu, H.; Dai, H. X. Tetrahedron Lett. 2019, 60, 151061. |
| [43] | Oost, R.; Misale, A.; Maulide, N. Angew. Chem. Int. Ed. 2016, 55, 4587. |
| [44] | Han, M. L.; Huang, W.; Liu, Y. W.; Liu, M.; Xu, H.; Xiong, H.; Dai, H. X. Org. Lett. 2021, 23, 172. |
| [45] | Huang, W.; Han, M. L.; Liu, Y. W.; Xu, H.; Dai, H. X. Chin. Chem. Lett. 2021, 32, 2765. |
| [46] | (a) Medina, J. M.; Moreno, J.; Racine, S.; Du, S.; Garg, N. K. Angew. Chem., Int. Ed. 2017, 56, 6567. |
| [46] | (b) Walker, J. A., Jr.; Vickerman, K. L.; Humke, J. N.; Stanley, L. M. J. Am. Chem. Soc. 2017, 139, 10228. |
| [46] | (c) Kadam, A. A.; Metz, T. L.; Qian, Y.; Stanley, L. M. ACS Catal. 2019, 9, 5651. |
| [46] | (d) Zheng, Y.-L.; Newman, S. G. Angew. Chem., Int. Ed. 2019, 58, 18159. |
| [46] | (e) Miles, K. C.; Le, C. C.; Stambuli, J. P. Chem. - Eur. J. 2014, 20, 11336. |
| [46] | (f) Zhao, X.; Tu, H.-Y.; Guo, L.; Zhu, S.; Qing, F.-L.; Chu, L. Nat. Commun. 2018, 9, 3488. |
| [47] | Liu, M.; Wang, X.; Guo, Z.; Li, H.; Huang, W.; Xu, H.; Dai, H.-X. Org. Lett. 2021, 23, 6299. |
| [48] | (a) Quasdorf, K. W.; Overman, L. E. Nature 2014, 516, 181. |
| [48] | (b) Zeng, X.-P.; Cao, Z.-Y.; Wang, Y.-H.; Zhou, F.; Zhou, J. Chem. Rev. 2016, 116, 7330. |
| [48] | (c) Long, R.; Huang, J.; Gong, J.; Yang, Z. Nat. Prod. Rep. 2015, 32, 1584. |
| [49] | (a) Catellani, M. Top. Organomet. Chem. 2005, 14, 21. |
| [49] | (b) Martins, A.; Mariampillai, B.; Lautens, M. Top. Curr. Chem. 2009, 292, 1. |
| [49] | (c) Ye, J.; Lautens, M. Nat. Chem. 2015, 7, 863. |
| [49] | (d) Cheng, H.-G.; Chen, S.-Q.; Chen, R.-M.; Zhou, Q.-H. Angew. Chem., Int. Ed. 2019, 58, 5832. |
| [49] | (e) Wang, J.-C.; Dong, G.-B. Chem. Rev. 2019, 119, 7478. |
| [50] | Sun, F.-G.; Li, M.; He, C.-F.; Wang, B.; Li, B.; Sui, X.-W.; Gu, Z.-H. J. Am. Chem. Soc. 2016, 138, 7456. |
| [51] | (a) Myers, A. G.; Tanaka, D.; Mannion, M. R. J. Am. Chem. Soc. 2002, 124, 11250. |
| [51] | (b) Goossen, L. J.; Deng, G.; Levy, L. M. Science 2006, 313, 662. |
| [51] | (c) Rodríguez, N.; Goossen, L. J. Chem. Soc. Rev. 2011, 40, 5030. |
| [52] | Han, M. L.; Chen, J. J.; Xu, H.; Huang, Z. C.; Huang, W.; Liu, Y. W.; Wang, X.; Liu, M.; Guo, Z. Q.; Dai, H. X. JACS Au 2021, 1, 1877. |
| [53] | (a) Tasker, S. Z.; Standley, E. A.; Jamison, T. F. Nature 2014, 509, 299. |
| [53] | (b) Ananikov, V. P. ACS Catal. 2015, 5, 1964. |
| [53] | (c) Diccianni, J. B.; Diao, T. Trends Chem. 2019, 1, 830. |
| [53] | (d) Chernyshev, V. M.; Ananikov, V. P. ACS Catal. 2022, 12, 1180. |
| [54] | Onaka, M.; Matsuoka, Y.; Mukaiyama, T. Chem. Lett. 1981, 10, 531. |
| [55] | Wotal, A. C.; Weix, D. J. Org. Lett. 2012, 14, 1476. |
| [56] | Wang, J.; Cary, B. P.; Beyer, P. D.; Gellman, S. H.; Weix, D. J. Angew. Chem. Int. Ed. 2019, 58, 12081. |
| [57] | Shimizu, T.; Seki, M. Tetrahedron Lett. 2002, 43, 1039. |
| [58] | Zhang, Y.; Rovis, T. J. Am. Chem. Soc. 2004, 126, 15964. |
| [59] | Villalobos, J. M.; Srogl, J.; Liebeskind, L. S. J. Am. Chem. Soc. 2007, 129, 15734. |
| [60] | Liebeskind, L. S.; Yang, H.; Li, H. Angew. Chem. Int. Ed. 2009, 48, 1417. |
| [61] | Zhang, Z. H.; Lindale, M. G.; Liebeskind, L. S. J. Am. Chem. Soc. 2011, 133, 6403. |
| [62] | Kobayashi, H.; Eickhoff, J. A.; Zakarian, A. J. Org. Chem. 2015, 80, 9989. |
| [63] | Ochiai, H.; Uetake, Y.; Niwa, T.; Hosoya, T. Angew. Chem., Int. Ed. 2017, 56, 2482. |
/
| 〈 |
|
〉 |