

Molecular Dynamics Simulation of Acetylene Pyrolysis into Fullerenes
Received date: 2023-02-09
Online published: 2023-04-25
Supported by
National Natural Science Foundation of China(51832008)
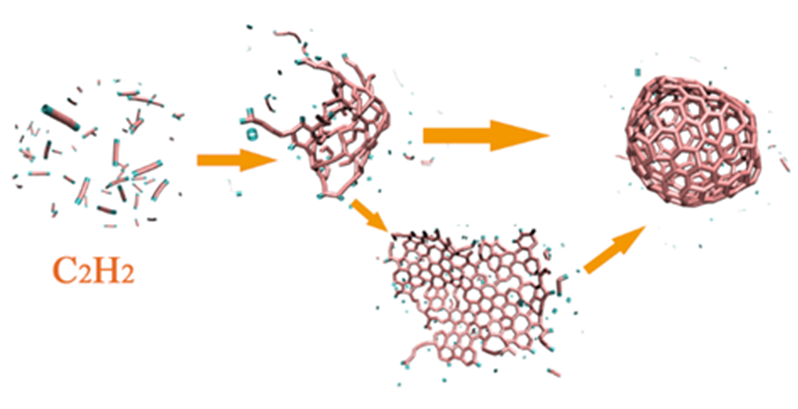
Acetylene (C2H2) is one of the main intermediate species in the industrial conversion of natural gas to many high-value chemicals. In this work, molecular dynamics simulation was carried out to study the possibility of producing fullerenes by high temperature pyrolysis of acetylene. The effect of atomic and molecular hydrogen, reaction temperature and carbon density were discussed according to the results obtained from reactive force filed molecular dynamics simulation. The results show that C2H2 can form fullerenes at suitable temperature and density. In the early stage, hydrogen in the system is conducive to the aggregation of small carbon clusters and the formation of carbon chains is faster. Interestingly, in the later stage, it is not conducive to further aggregation reaction, but the atomic and molecular hydrogen produced by C2H2 pyrolysis can make the carbon clusters rounder and prevent the excessive growth of the carbon clusters, and can make the carbon cage more similar to classical fullerenes, which suggest that the production of fullerenes from C2H2 does not require the assistance of inert gases, thus significantly reducing the production cost of fullerenes in principle. Two main pathways of fullerenes formation were observed in simulations under different conditions. The first pathway is from carbon chain, cluster to carbon cage, most occurring in low density conditions. The other pathway is from carbon chain, cluster, graphene and carbon cage, most occurring in high density conditions. The growth of six-membered rings during fullerene formation is realized mainly via carbon cage defects and bond rotation, and the general trend is to make the carbon cage closer to the classical fullerene. The preparation of fullerenes by C2H2 pyrolysis is worthy of further study. These results are helpful to understand the process of producing fullerenes from the pyrolysis of C2H2 and have important implications for the development of industrial production methods of fullerenes.
Key words: acetylene; pyrolysis; fullerene; molecular dynamics; formation mechanism
Zhenyu Liu , Li-Hua Gan . Molecular Dynamics Simulation of Acetylene Pyrolysis into Fullerenes[J]. Acta Chimica Sinica, 2023 , 81(5) : 502 -510 . DOI: 10.6023/A23020026
| [1] | Xie, S.-Y.; Yang, S.-F.; Li, S.-H. Fullerenes: Fundamental and Application,Science Press, Beijing, 2019. (in Chinese) |
| [1] | (谢素原, 杨上峰, 李姝慧, 富勒烯: 从基础到应用, 科学出版社, 北京, 2019.) |
| [2] | Gan, L.-H.; Wang, C.-R. Structure, Properties and Applications of Fullerenes and Their Derivatives, Chemical Industry Press, Beijing, 2019. (in Chinese) |
| [2] | (甘利华, 王春儒, 富勒烯及其衍生物的结构、性质和应用, 化学工业出版社, 北京, 2019.) |
| [3] | Qiu, L.; Liang, J.-Y.; Zhang, Z.-X.; Wang, T.-S. Acta Chim. Sinica 2022, 80, 874. (in Chinese) |
| [3] | (邱玲, 梁家艺, 张竹霞, 王太山, 化学学报, 2022, 80, 874.) |
| [4] | Wu, B.; Wang, C.; Li, B.-L.; Wang, C.-R. Acta Chim. Sinica 2022, 80, 101. (in Chinese) |
| [4] | (吴波, 王冲, 李宝林, 王春儒, 化学学报, 2022, 80, 101.) |
| [5] | Ramazani, A.; Moghaddasi, M. A.; Malekzadeh, A. M.; Rezayati, S.; Hanifehpour, Y.; Joo, S. W. Inorg. Chem. Commun. 2021, 125, 108442. |
| [6] | Xue, X.-G.; Meng, L.-Y.; Ma, Y.; Zhang, C.-Y. J. Phys. Chem. C 2017, 121, 7502. |
| [7] | Xu, H. M.S. Thesis, Southwest University, Chongqing, 2020. (in Chinese) |
| [7] | (徐惠, 硕士论文, 西南大学, 重庆, 2020.) |
| [8] | Howard, J. B.; McKinnon, J. T.; Johnson, M. E.; Makarovsky, Y.; Lafleur, A. L. J. Phys. Chem. 1992, 96, 6657. |
| [9] | Homann, K. H. Angew. Chem., Int. Ed. 1998, 37, 2434. |
| [10] | Takehara, H.; Fujiwara, M.; Arikawa, M.; Diener, M. D.; Alford, J. M. Carbon 2005, 43, 311. |
| [11] | Zhu, Y.; Zhang, G.; Zhang, W.; Lin, T.; Xie, H.; Liu, Q.; Zhang, H. J. Wuhan Univ. Technol. (Mater. Sci. Ed.) 2007, 22, 94. |
| [12] | Sharma, A.; Mukut, K. M.; Roy, S. P.; Goudeli, E. Carbon 2021, 180, 215. |
| [13] | Wang, Y.; Gu, M.-Y.; Wu, J.-J.; Cao, L.; Lin, Y.-Y.; Huang, X. Y. Int. J. Hydrogen Energy 2021, 46, 36557. |
| [14] | Zhao, J.; Lin, Y.-Y.; Huang, K.; Gu, M.-Y.; Lu, K.; Chen, P.; Wang, Y.; Zhu, B.-C. Fuel 2020, 262, 116677. |
| [15] | Han, S.; Li, X.; Nie, F.; Zheng, M.; Liu, X.; Guo, L. Energy Fuels 2017, 31, 8434. |
| [16] | Liu, Y.; Wei, X.; Sun, W.-Z.; Zhao, L. Energy Fuels 2021, 35, 16778. |
| [17] | Zhang, C.-Y.; Zhang, C.; Ma, Y.; Xue, X.-G. Phys. Chem. Chem. Phys. 2015, 17, 11469. |
| [18] | Zhong, R.; Hong, R. Chem. Eng. J. 2020, 387, 124102. |
| [19] | Li, H. B.; Page, A. J.; Irle, S.; Morokuma, K. J. Phys. Chem. Lett. 2013, 4, 2323. |
| [20] | Van Duin, A. C.; Dasgupta, S.; Lorant, F.; Goddard, W. A. J. Phys. Chem. A 2001, 105, 9396. |
| [21] | Brenner, D. W. Phys. Rev. B 1990, 42, 9458. |
| [22] | Mao, Q.; Van Duin, A. C.; Luo, K. H. Carbon 2017, 121, 380. |
| [23] | Yoon, K.; Rahnamoun, A.; Swett, J. L.; Iberi, V.; Cullen, D. A.; Vlassiouk, I. V.; Belianinov, A.; Jesse, S.; Sang, X.; Ovchinnikova, O. S.; Rondinone, A. J.; Unocic, R. R.; Van Duin, A. C. ACS Nano. 2016, 10, 8376. |
| [24] | Chen, J.; Pei, J.; Zhao, H. J. Phys. Chem. C 2021, 125, 19345. |
| [25] | Mei, H.; Cui, J.; He, X.; Lu, Y.; Sun, X.; Xu, K.; Mei, X. J. Phys. Chem. C 2022, 126, 13388. |
| [26] | Gaikwad, P. S.; Kowalik, M.; Jensen, B. D.; Van Duin, A.; Odegard, G. M. ACS Appl. Nano Mater. 2022, 5, 5915. |
| [27] | Qian, H. J.; van Duin, A. C.; Morokuma, K.; Irle, S. J. Chem. Theory Comput. 2011, 7, 2040. |
| [28] | Thompson, A. P.; Aktulga, H. M.; Berger, R.; Bolintineanu, D. S.; Brown, W. M.; Crozier, P. S.; in't Veld, P. J.; Kohlmeyer, A.; Moore, S. G.; Nguyen, T. D.; Shan, R.; Stevens, M. J.; Tranchida, J.; Trott, C.; Plimpton, S. J. Comp. Phys. Commun. 2022, 271, 108171. |
| [29] | Plimpton, S. J. Comput. Phys. 1995, 117, 1. |
| [30] | Hoover, W. G. Phys. Rev. A 1985, 31, 1695. |
| [31] | Humphrey, W.; Dalke, A.; Schulten, K. J. Mol. Graphics 1996, 14, 33. |
| [32] | Qian, H. J.; Wang, Y.; Morokuma, K. Carbon 2017, 114, 635. |
| [33] | Ma, J.; Chen, X.; Song, M.; Wang, C.; Xia, W. Diamond Relat. Mater. 2021, 117, 108445. |
| [34] | Saha, B.; Irle, S.; Morokuma, K. J. Phys. Chem. C 2011, 115, 22707. |
/
| 〈 |
|
〉 |