Acta Chimica Sinica ›› 2023, Vol. 81 ›› Issue (5): 502-510.DOI: 10.6023/A23020026 Previous Articles Next Articles
Article
投稿日期:2023-02-09
发布日期:2023-04-24
基金资助:Received:2023-02-09
Published:2023-04-24
Contact:
*E-mail: ganlh@swu.edu.cn
Supported by:Share
Zhenyu Liu, Li-Hua Gan. Molecular Dynamics Simulation of Acetylene Pyrolysis into Fullerenes[J]. Acta Chimica Sinica, 2023, 81(5): 502-510.
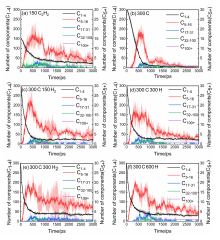

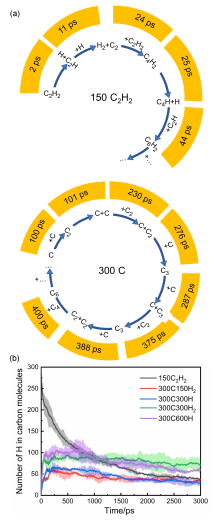

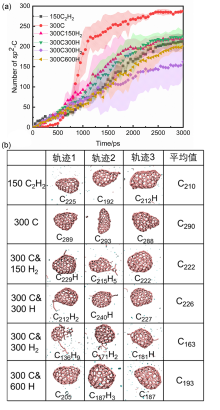

| System | N5 | N6 | N5+N6 | N3-8 | N6/N3-8 | (N5+N6)/N3-8 |
|---|---|---|---|---|---|---|
| 150 C2H2 | 24 | 67 | 91 | 108 | 0.620 | 0.843 |
| 300 C | 34 | 89 | 123 | 146 | 0.610 | 0.842 |
| 300 C&300 H | 24 | 74 | 98 | 112 | 0.661 | 0.875 |
| 300 C&300 H2 | 18 | 47 | 65 | 75 | 0.627 | 0.867 |
| 300 C&600 H | 22 | 65 | 87 | 98 | 0.663 | 0.888 |
| System | N5 | N6 | N5+N6 | N3-8 | N6/N3-8 | (N5+N6)/N3-8 |
|---|---|---|---|---|---|---|
| 150 C2H2 | 24 | 67 | 91 | 108 | 0.620 | 0.843 |
| 300 C | 34 | 89 | 123 | 146 | 0.610 | 0.842 |
| 300 C&300 H | 24 | 74 | 98 | 112 | 0.661 | 0.875 |
| 300 C&300 H2 | 18 | 47 | 65 | 75 | 0.627 | 0.867 |
| 300 C&600 H | 22 | 65 | 87 | 98 | 0.663 | 0.888 |
| 密度及编号 | 出现时间 | 组成 | 消失时间 | 组成 | 存在时长 | H/C变化 |
|---|---|---|---|---|---|---|
| 5-4 | 1.450 | C90H5 | 1.542 | C122H8 | 0.092 | 0.010 |
| 25-2 | 0.540 | C124H17 | 0.640 | C112H11 | 0.100 | -0.039 |
| 25-5 | 0.540 | C200H24 | 0.825 | C247H32 | 0.285 | 0.010 |
| 5-5 | 1.300 | C108H5 | 1.850 | C183H7 | 0.550 | -0.008 |
| 50-5 | 0.500 | C229H39 | 1.260 | C283H26 | 0.760 | -0.078 |
| 50-4 | 0.550 | C233H39 | 1.510 | C284H17 | 0.960 | -0.108 |
| 25-7 | 0.790 | C162H21 | 1.800 | C262H14 | 1.010 | -0.076 |
| 25-3 | 0.580 | C150H22 | 1.680 | C277H19 | 1.100 | -0.078 |
| 50-2 | 0.620 | C204H38 | 2.050 | C285H18 | 1.430 | -0.123 |
| 25-0 | 0.750 | C229H36 | 2.190 | C283H19 | 1.440 | -0.090 |
| 25-4 | 0.617 | C162H24 | 2.065 | C279H17 | 1.448 | -0.087 |
| 50-9 | 0.540 | C212H43 | 1.990 | C283H24 | 1.450 | -0.118 |
| 25-8 | 0.700 | C199H29 | 5.000 | C295H14 | 4.300 | -0.098 |
| 密度及编号 | 出现时间 | 组成 | 消失时间 | 组成 | 存在时长 | H/C变化 |
|---|---|---|---|---|---|---|
| 5-4 | 1.450 | C90H5 | 1.542 | C122H8 | 0.092 | 0.010 |
| 25-2 | 0.540 | C124H17 | 0.640 | C112H11 | 0.100 | -0.039 |
| 25-5 | 0.540 | C200H24 | 0.825 | C247H32 | 0.285 | 0.010 |
| 5-5 | 1.300 | C108H5 | 1.850 | C183H7 | 0.550 | -0.008 |
| 50-5 | 0.500 | C229H39 | 1.260 | C283H26 | 0.760 | -0.078 |
| 50-4 | 0.550 | C233H39 | 1.510 | C284H17 | 0.960 | -0.108 |
| 25-7 | 0.790 | C162H21 | 1.800 | C262H14 | 1.010 | -0.076 |
| 25-3 | 0.580 | C150H22 | 1.680 | C277H19 | 1.100 | -0.078 |
| 50-2 | 0.620 | C204H38 | 2.050 | C285H18 | 1.430 | -0.123 |
| 25-0 | 0.750 | C229H36 | 2.190 | C283H19 | 1.440 | -0.090 |
| 25-4 | 0.617 | C162H24 | 2.065 | C279H17 | 1.448 | -0.087 |
| 50-9 | 0.540 | C212H43 | 1.990 | C283H24 | 1.450 | -0.118 |
| 25-8 | 0.700 | C199H29 | 5.000 | C295H14 | 4.300 | -0.098 |
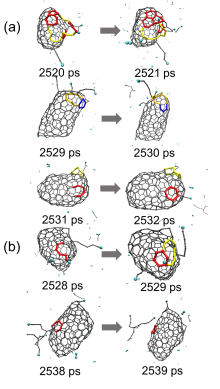

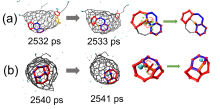
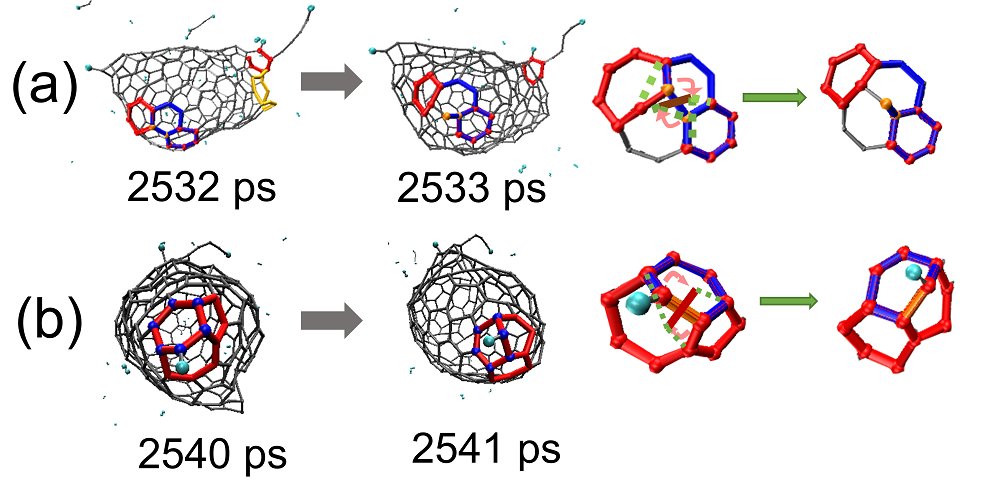
| Time/ps | δN6 | 缺口收缩/扩大 | 碳链 | 键旋转 |
|---|---|---|---|---|
| 2520~2521 | 5 | 5 | ||
| 2521~2522 | -2 | -2 | ||
| 2523~2524 | -1 | -1 | ||
| 2524~2525 | 2 | 2 | ||
| 2526~2527 | -2 | -2 | ||
| 2527~2528 | 2 | 2 | ||
| 2528~2529 | 1 | 1 | ||
| 2529~2530 | -1 | -1 | ||
| 2530~2531 | 1 | 2 | -1 | |
| 2531~2532 | 0 | |||
| 2532~2533 | 1 | 1 | ||
| 2534~2535 | -1 | -2 | 1 | |
| 2535~2536 | 3 | 2 | 1 | |
| 2536~2537 | -2 | -2 | ||
| 2537~2538 | 1 | |||
| 2538~2539 | 1 | 2 | ||
| 2539~2540 | -1 | -1 | ||
| 2540~2541 | 3 | 3 | ||
| 总计 | 10 | 6 | 1 | 3 |
| Time/ps | δN6 | 缺口收缩/扩大 | 碳链 | 键旋转 |
|---|---|---|---|---|
| 2520~2521 | 5 | 5 | ||
| 2521~2522 | -2 | -2 | ||
| 2523~2524 | -1 | -1 | ||
| 2524~2525 | 2 | 2 | ||
| 2526~2527 | -2 | -2 | ||
| 2527~2528 | 2 | 2 | ||
| 2528~2529 | 1 | 1 | ||
| 2529~2530 | -1 | -1 | ||
| 2530~2531 | 1 | 2 | -1 | |
| 2531~2532 | 0 | |||
| 2532~2533 | 1 | 1 | ||
| 2534~2535 | -1 | -2 | 1 | |
| 2535~2536 | 3 | 2 | 1 | |
| 2536~2537 | -2 | -2 | ||
| 2537~2538 | 1 | |||
| 2538~2539 | 1 | 2 | ||
| 2539~2540 | -1 | -1 | ||
| 2540~2541 | 3 | 3 | ||
| 总计 | 10 | 6 | 1 | 3 |
| [1] |
Xie, S.-Y.; Yang, S.-F.; Li, S.-H. Fullerenes: Fundamental and Application,Science Press, Beijing, 2019. (in Chinese)
|
|
(谢素原, 杨上峰, 李姝慧, 富勒烯: 从基础到应用, 科学出版社, 北京, 2019.)
|
|
| [2] |
Gan, L.-H.; Wang, C.-R. Structure, Properties and Applications of Fullerenes and Their Derivatives, Chemical Industry Press, Beijing, 2019. (in Chinese)
|
|
(甘利华, 王春儒, 富勒烯及其衍生物的结构、性质和应用, 化学工业出版社, 北京, 2019.)
|
|
| [3] |
Qiu, L.; Liang, J.-Y.; Zhang, Z.-X.; Wang, T.-S. Acta Chim. Sinica 2022, 80, 874. (in Chinese)
doi: 10.6023/A22020087 |
|
(邱玲, 梁家艺, 张竹霞, 王太山, 化学学报, 2022, 80, 874.)
doi: 10.6023/A22020087 |
|
| [4] |
Wu, B.; Wang, C.; Li, B.-L.; Wang, C.-R. Acta Chim. Sinica 2022, 80, 101. (in Chinese)
doi: 10.6023/A21120564 |
|
(吴波, 王冲, 李宝林, 王春儒, 化学学报, 2022, 80, 101.)
doi: 10.6023/A21120564 |
|
| [5] |
Ramazani, A.; Moghaddasi, M. A.; Malekzadeh, A. M.; Rezayati, S.; Hanifehpour, Y.; Joo, S. W. Inorg. Chem. Commun. 2021, 125, 108442.
doi: 10.1016/j.inoche.2021.108442 |
| [6] |
Xue, X.-G.; Meng, L.-Y.; Ma, Y.; Zhang, C.-Y. J. Phys. Chem. C 2017, 121, 7502.
doi: 10.1021/acs.jpcc.7b00294 |
| [7] |
Xu, H. M.S. Thesis, Southwest University, Chongqing, 2020. (in Chinese)
|
|
(徐惠, 硕士论文, 西南大学, 重庆, 2020.)
|
|
| [8] |
Howard, J. B.; McKinnon, J. T.; Johnson, M. E.; Makarovsky, Y.; Lafleur, A. L. J. Phys. Chem. 1992, 96, 6657.
doi: 10.1021/j100195a026 |
| [9] |
Homann, K. H. Angew. Chem., Int. Ed. 1998, 37, 2434.
doi: 10.1002/(ISSN)1521-3773 |
| [10] |
Takehara, H.; Fujiwara, M.; Arikawa, M.; Diener, M. D.; Alford, J. M. Carbon 2005, 43, 311.
doi: 10.1016/j.carbon.2004.09.017 |
| [11] |
Zhu, Y.; Zhang, G.; Zhang, W.; Lin, T.; Xie, H.; Liu, Q.; Zhang, H. J. Wuhan Univ. Technol. (Mater. Sci. Ed.) 2007, 22, 94.
|
| [12] |
Sharma, A.; Mukut, K. M.; Roy, S. P.; Goudeli, E. Carbon 2021, 180, 215.
doi: 10.1016/j.carbon.2021.04.065 |
| [13] |
Wang, Y.; Gu, M.-Y.; Wu, J.-J.; Cao, L.; Lin, Y.-Y.; Huang, X. Y. Int. J. Hydrogen Energy 2021, 46, 36557.
doi: 10.1016/j.ijhydene.2021.08.125 |
| [14] |
Zhao, J.; Lin, Y.-Y.; Huang, K.; Gu, M.-Y.; Lu, K.; Chen, P.; Wang, Y.; Zhu, B.-C. Fuel 2020, 262, 116677.
doi: 10.1016/j.fuel.2019.116677 |
| [15] |
Han, S.; Li, X.; Nie, F.; Zheng, M.; Liu, X.; Guo, L. Energy Fuels 2017, 31, 8434.
doi: 10.1021/acs.energyfuels.7b01194 |
| [16] |
Liu, Y.; Wei, X.; Sun, W.-Z.; Zhao, L. Energy Fuels 2021, 35, 16778.
doi: 10.1021/acs.energyfuels.1c02462 |
| [17] |
Zhang, C.-Y.; Zhang, C.; Ma, Y.; Xue, X.-G. Phys. Chem. Chem. Phys. 2015, 17, 11469.
doi: 10.1039/C5CP00926J |
| [18] |
Zhong, R.; Hong, R. Chem. Eng. J. 2020, 387, 124102.
doi: 10.1016/j.cej.2020.124102 |
| [19] |
Li, H. B.; Page, A. J.; Irle, S.; Morokuma, K. J. Phys. Chem. Lett. 2013, 4, 2323.
doi: 10.1021/jz400925f |
| [20] |
Van Duin, A. C.; Dasgupta, S.; Lorant, F.; Goddard, W. A. J. Phys. Chem. A 2001, 105, 9396.
doi: 10.1021/jp004368u |
| [21] |
Brenner, D. W. Phys. Rev. B 1990, 42, 9458.
doi: 10.1103/PhysRevB.42.9458 |
| [22] |
Mao, Q.; Van Duin, A. C.; Luo, K. H. Carbon 2017, 121, 380.
doi: 10.1016/j.carbon.2017.06.009 |
| [23] |
Yoon, K.; Rahnamoun, A.; Swett, J. L.; Iberi, V.; Cullen, D. A.; Vlassiouk, I. V.; Belianinov, A.; Jesse, S.; Sang, X.; Ovchinnikova, O. S.; Rondinone, A. J.; Unocic, R. R.; Van Duin, A. C. ACS Nano. 2016, 10, 8376.
doi: 10.1021/acsnano.6b03036 |
| [24] |
Chen, J.; Pei, J.; Zhao, H. J. Phys. Chem. C 2021, 125, 19345.
doi: 10.1021/acs.jpcc.1c02610 |
| [25] |
Mei, H.; Cui, J.; He, X.; Lu, Y.; Sun, X.; Xu, K.; Mei, X. J. Phys. Chem. C 2022, 126, 13388.
doi: 10.1021/acs.jpcc.2c02784 |
| [26] |
Gaikwad, P. S.; Kowalik, M.; Jensen, B. D.; Van Duin, A.; Odegard, G. M. ACS Appl. Nano Mater. 2022, 5, 5915.
doi: 10.1021/acsanm.2c01280 |
| [27] |
Qian, H. J.; van Duin, A. C.; Morokuma, K.; Irle, S. J. Chem. Theory Comput. 2011, 7, 2040.
doi: 10.1021/ct200197v |
| [28] |
Thompson, A. P.; Aktulga, H. M.; Berger, R.; Bolintineanu, D. S.; Brown, W. M.; Crozier, P. S.; in't Veld, P. J.; Kohlmeyer, A.; Moore, S. G.; Nguyen, T. D.; Shan, R.; Stevens, M. J.; Tranchida, J.; Trott, C.; Plimpton, S. J. Comp. Phys. Commun. 2022, 271, 108171.
doi: 10.1016/j.cpc.2021.108171 |
| [29] |
Plimpton, S. J. Comput. Phys. 1995, 117, 1.
|
| [30] |
Hoover, W. G. Phys. Rev. A 1985, 31, 1695.
doi: 10.1103/PhysRevA.31.1695 |
| [31] |
Humphrey, W.; Dalke, A.; Schulten, K. J. Mol. Graphics 1996, 14, 33.
doi: 10.1016/0263-7855(96)00018-5 |
| [32] |
Qian, H. J.; Wang, Y.; Morokuma, K. Carbon 2017, 114, 635.
doi: 10.1016/j.carbon.2016.12.062 |
| [33] |
Ma, J.; Chen, X.; Song, M.; Wang, C.; Xia, W. Diamond Relat. Mater. 2021, 117, 108445.
doi: 10.1016/j.diamond.2021.108445 |
| [34] |
Saha, B.; Irle, S.; Morokuma, K. J. Phys. Chem. C 2011, 115, 22707.
doi: 10.1021/jp203614e |
| [1] | Yizhi Han, Jianhui Lan, Xue Liu, Weiqun Shi. Advances in Molecular Dynamics Studies of Molten Salts Based on Machine Learning [J]. Acta Chimica Sinica, 2023, 81(11): 1663-1672. |
| [2] | Ke Zhao, Xiayu Cheng, Xuexue Ma, Minghui Geng. Mechanism of Two-photon Absorption Enhancement for a Piperazine-based Zinc Ion Probe [J]. Acta Chimica Sinica, 2023, 81(10): 1371-1378. |
| [3] | Yuguang Sui, Jinrong Zhou, Pan Liao, Wenjie Liang, Hai Xu. A Gaint Donor-Acceptor Molecular Switch Compound: Synthesis and Properties [J]. Acta Chimica Sinica, 2022, 80(8): 1061-1065. |
| [4] | Ling Qiu, Jiayi Liang, Zhuxia Zhang, Taishan Wang. Synthesis and Characterizations of 15N Isotope Labeling Metal Nitride Clusterfullerene [J]. Acta Chimica Sinica, 2022, 80(7): 874-878. |
| [5] | Dan Wang, Bo Feng, Xiaoxin Zhang, Yanan Liu, Yan Pei, Minghua Qiao, Baoning Zong. Nitrogen-doped Carbon Pyrolyzed from ZIF-8 for Electrocatalytic Oxygen Reduction to Hydrogen Peroxide [J]. Acta Chimica Sinica, 2022, 80(6): 772-780. |
| [6] | Wenyuan Lin, Qingzhe Zhu, Yunlong Ma, Peng Wang, Shuo Wan, Qingdong Zheng. Rationally Tuning Blend Miscibility of Polymer Donor and Nonfullerene Acceptor for Constructing Efficient Organic Solar Cells※ [J]. Acta Chimica Sinica, 2022, 80(6): 724-733. |
| [7] | Bo Wu, Chong Wang, Baolin Li, Chunru Wang. Light-driven Molecular Magnetic Switch for a Metallofullerene※ [J]. Acta Chimica Sinica, 2022, 80(2): 101-104. |
| [8] | Yingzhe Du, Heng Zhang, Shiling Yuan. Molecular Dynamics Simulation of Thermal Conductivity of Al2O3/PDMS Composites [J]. Acta Chimica Sinica, 2021, 79(6): 787-793. |
| [9] | Chang-An Liu, Shi-Bo Hong, Bei Li. Molecular Dynamics Simulation of the Stability Behavior of Graphene in Glycerol/Urea Solvents in Liquid-Phase Exfoliation [J]. Acta Chimica Sinica, 2021, 79(4): 530-538. |
| [10] | Haohao Fu, Haochuan Chen, Hong Zhang, Xueguang Shao, Wensheng Cai. Accurate Estimation of Protein-ligand Binding Free Energies Based on Geometric Restraints [J]. Acta Chimica Sinica, 2021, 79(4): 472-480. |
| [11] | Zun Liang, Xin Zhang, Songtai Lv, Hongtao Liang, Yang Yang. Crystal-Melt Interface Kinetics and the Capillary Wave Dynamics of the Monolayer Confined Ice-Water Coexistence Lines [J]. Acta Chimica Sinica, 2021, 79(1): 108-118. |
| [12] | Fan Qin, Liang Hongtao, Xu Xianqi, Lv Songtai, Liang Zun, Yang Yang. Study of the Dielectric Property of Monolayer Confined Water Using A Polarizable Model [J]. Acta Chimica Sinica, 2020, 78(6): 547-556. |
| [13] | Gao Simeng, Xia Kun, Kang Zhihong, Nai Yongning, Yuan Ruixia, Niu Ruixia. Molecular Dynamics Simulation of “Quasi-Gemini” Anionic Surfactant at the Decane/Water Interface [J]. Acta Chimica Sinica, 2020, 78(2): 155-160. |
| [14] | Hu Yuhui, Wu Wenlin, Yu Liyang, Luo Kaijun, Xu Xiaopeng, Li Ying, Peng Qiang. Synthesis and Photovoltaic Properties of Perylene Diimide Based Small Molecular Acceptors with a Diketopyrrolopyrrole Core [J]. Acta Chimica Sinica, 2020, 78(11): 1246-1254. |
| [15] | Yang, Pengli, Wang, Zhenxing, Liang, Zun, Liang, Hongtao, Yang, Yang. A Molecular Dynamics Simulation Study of the Effect of External Electric Field on the Water Surface Potential [J]. Acta Chimica Sinica, 2019, 77(10): 1045-1053. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
