

Fabrication and Mechanism Study of Clustered Au/CeO2 Catalyst for the CO Oxidation Reaction★
Received date: 2023-04-28
Online published: 2023-06-12
Supported by
National Key Research and Development Program of China(2021YFA1501103); National Science Fund for Distinguished Young Scholars of China(22225110); National Natural Science Foundation of China(22075166); National Natural Science Foundation of China(22271177); Young Scholars Program of Shandong University
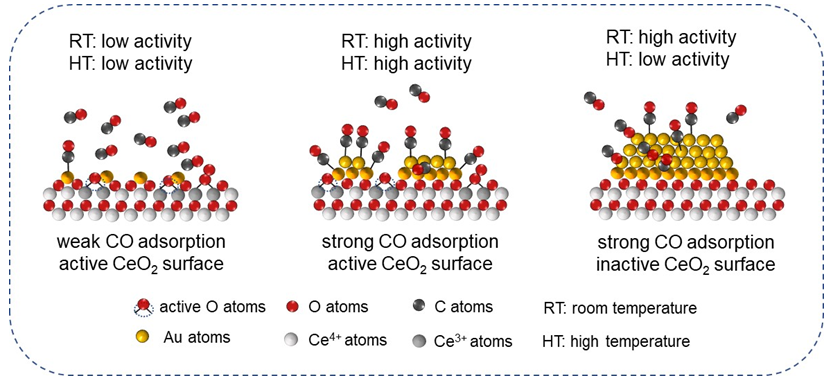
Supported Au-based catalysts have been attracting continuous attention owing to their outstanding performance in various catalytic applications. However, the complicated environment on the catalyst surface severely hampered the unambiguous illustration of the structural-function relationship for Au-based catalysts. In this work, we developed a facile strategy to fabricate various Au species [Aun+ (n>1), Auδ+ (0<n<1) and Au0] onto the CeO2 support, which the Au/CeO2 interaction was distinctively modulated by the CeO2-x with different calcination temperatures. As-prepared Au/CeO2-x were valued as catalysts for CO oxidation reaction, which is important for both environmental application and model catalysis. The as-formed clustered Au with δ+ oxidation state on CeO2-400 demonstrates the best catalytic performance at 50 ℃, while the Au nanoparticles with dominant Au0 atoms are superior for catalyzing CO oxidation at room temperature. However, the monodispersed Aun+ single-sites with the highest dispersion are almost inactive for CO oxidation below 50 ℃. On the basis of structural characterizations and in-situ diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) results, we reasonably speculated that the electronic state of Au species plays a predominant role at room temperature for catalytic performance owing to the differentiated CO adsorption ability. This speculation is well in line with our former research finding that the Aun+ sites are weak in capturing CO molecules and Au0 is favorable for CO adsorption. The surficial O atoms, especially the lattice O atoms, play a minor role in catalyzing CO oxidation for the Au particles within Au/CeO2-700, implying that the reactant molecules might prefer a L-H pathway at room temperature. In contrast, the clustered Auδ+ with moderate CO adsorption ability and abundant interfacial site was apt to participate in the surface reaction with a MvK pathway, in which the reaction between surficial lattice O atoms and adsorbed CO molecules significantly contribute to the catalytic activity. Therefore, the Au/CeO2-400 catalyst coupling with moderate CO adsorption ability and abundant active O atoms displayed the best catalytic efficiency at elevated temperatures. These findings in this work provided a facile route for the fabrication efficient Au/CeO2 catalyst, and shed light on the molecular understanding of the reaction path over various Au sites.
Xinpu Fu , Xiuling Wang , Weiwei Wang , Rui Si , Chunjiang Jia . Fabrication and Mechanism Study of Clustered Au/CeO2 Catalyst for the CO Oxidation Reaction★[J]. Acta Chimica Sinica, 2023 , 81(8) : 874 -883 . DOI: 10.6023/A23040174
| [1] | Haruta M.; Kobayashi T.; Sano H.; Yamada N. Chem. Lett. 1987, 16, 405. |
| [2] | Hutchings G. J. J. Catal. 1985, 96, 292. |
| [3] | Jin Z.; Song Y. Y.; Fu X. P.; Song Q. S.; Jia C. J. Chin. J. Chem. 2018, 36, 639. |
| [4] | Camellone M. F.; Fabris S. J. Am. Chem. Soc. 2009, 131, 10473. |
| [5] | Saavedra J.; Doan H. A.; Pursell C. J.; Grabow L. C.; Chandler B. D. Science 2014, 345, 1599. |
| [6] | Liu L.; Corma A. Chem. Rev. 2018, 118, 4981. |
| [7] | Ha H.; Yoon S.; An K.; Kim H. Y. ACS Catal. 2018, 8, 11491. |
| [8] | Lee Y.; He G.; Akey A. J.; Si R.; Flytzani-Stephanopoulos M.; Herman I. P. J. Am. Chem. Soc. 2011, 133, 12952. |
| [9] | Boccuzzi F.; Chiorino A.; Manzoli M.; Lu P.; Akita T.; Ichikawa S.; Haruta M. J. Catal. 2001, 202, 256. |
| [10] | Guo L.-W.; Du P.-P.; Fu X.-P.; Ma C.; Zeng J.; Si R.; Huang Y.-Y.; Jia C.-J.; Zhang Y.-W.; Yan C.-H. Nat. Commun. 2016, 7, 1. |
| [11] | Schlexer P.; Widmann D.; Behm R. J.; Pacchioni G. ACS Catal. 2018, 8, 6513. |
| [12] | Rodriguez J. A.; Grinter D. C.; Liu Z.; Palomino R. M.; Senanayake S. D. Chem. Soc. Rev. 2017, 46, 1824. |
| [13] | Ke J.; Zhu W.; Jiang Y.; Si R.; Wang Y.-J.; Li S.-C.; Jin C.; Liu H.; Song W.-G.; Yan C.-H.; Zhang Y.-W. ACS Catal. 2015, 5, 5164. |
| [14] | Teng B.-C.; Jiang S.-Y.; Guo X.-W.; Yuan J.-H.; Luo M.-F. Acta Chim. Sinica 2009, 67, 2765. (in Chinese) |
| [14] | ( 滕波涛, 蒋仕宇, 郭晓伟, 袁金焕, 罗孟飞, 化学学报, 2009, 67, 2765.) |
| [15] | Jiao T.; Xu X.-L.; Zhang L.; Weng Y.-Y.; Weng Y.-B.; Gao Z.-X. Acta Chim. Sinica 2021, 79, 513. (in Chinese) |
| [15] | ( 焦桐, 许雪莲, 张磊, 翁幼云, 翁玉冰, 高志贤, 化学学报, 2021, 79, 513.) |
| [16] | Lin J.; Zhang L.; Ni J.; Wang R.; Wei K. Acta Chim. Sinica 2012, 70, 137. (in Chinese) |
| [16] | ( 林建新, 张留明, 倪军, 王榕, 魏可镁, 化学学报, 2012, 70, 137.) |
| [17] | Spezzati G.; Benavidez A. D.; DeLaRiva A. T.; Su Y.; Hofmann J. P.; Asahina S.; Olivier E. J.; Neethling J. H.; Miller J. T.; Datye A. K.; Hensen E. J. M. Appl. Catal. B: Environ. 2019, 243, 36. |
| [18] | Sun X.-C.; Yuan K.; Hua W.-D.; Gao Z.-R.; Zhang Q.; Yuan C.-Y.; Liu H.-C.; Zhang Y.-W. ACS Catal. 2022, 12, 11942. |
| [19] | Si R.; Flytzani-Stephanopoulos M. Angew. Chem. Int. Ed. 2008, 47, 2884. |
| [20] | Zhang S.; Lee J.; Kim D. H.; Kim T. Molecular Catalysis 2020, 482. |
| [21] | Yu W.-Z.; Wang W.-W.; Li S.-Q.; Fu X.-P.; Wang X.; Wu K.; Si R.; Ma C.; Jia C.-J.; Yan C.-H. J. Am. Chem. Soc. 2019, 141, 17548. |
| [22] | Manibalan G.; Murugadoss G.; Thangamuthu R.; Kumar R. M.; Jayavel R.; Kumar M. R. Materials Research Express 2019, 6, 075032. |
| [23] | Mihaylov M.; Kn?zinger H.; Hadjiivanov K.; Gates B. C. Chem. Ing. Tech. 2007, 79, 795. |
| [24] | McEntee M.; Stevanovic A.; Tang W.; Neurock M.; Yates J. T. Jr. J. Am. Chem. Soc. 2015, 137, 1972. |
| [25] | Zhang S.; Li X.-S.; Chen B.; Zhu X.; Shi C.; Zhu A.-M. ACS Catal. 2014, 4, 3481. |
| [26] | El-Moemen A. A.; Ku?erová G.; Behm R. J. Appl. Catal. B: Environ. 2010, 95, 57. |
| [27] | Kang L.; Wang B.; Bing Q.; Zalibera M.; Buchel R.; Xu R.; Wang Q.; Liu Y.; Gianolio D.; Tang C. C.; Gibson E. K.; Danaie M.; Allen C.; Wu K.; Marlow S.; Sun L. D.; He Q.; Guan S.; Savitsky A.; Velasco-Velez J. J.; Callison J.; Kay C. W. M.; Pratsinis S. E.; Lubitz W.; Liu J. Y.; Wang F. R. Nat. Commun. 2020, 11, 4008. |
| [28] | Kim H. Y.; Lee H. M.; Henkelman G. J. Am. Chem. Soc. 2012, 134, 1560. |
/
| 〈 |
|
〉 |