Acta Chimica Sinica ›› 2023, Vol. 81 ›› Issue (8): 874-883.DOI: 10.6023/A23040174 Previous Articles Next Articles
Special Issue: 庆祝《化学学报》创刊90周年合辑
Article
付信朴a, 王秀玲a, 王伟伟a, 司锐b, 贾春江a,*( )
)
投稿日期:2023-04-28
发布日期:2023-09-14
作者简介:基金资助:
Xinpu Fua, Xiuling Wanga, Weiwei Wanga, Rui Sib, Chunjiang Jiaa( )
)
Received:2023-04-28
Published:2023-09-14
Contact:
*E-mail: jiacj@sdu.edu.cn
About author:Supported by:Share
Xinpu Fu, Xiuling Wang, Weiwei Wang, Rui Si, Chunjiang Jia. Fabrication and Mechanism Study of Clustered Au/CeO2 Catalyst for the CO Oxidation Reaction★[J]. Acta Chimica Sinica, 2023, 81(8): 874-883.
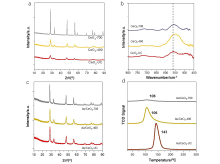

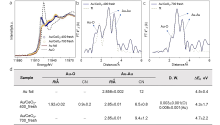



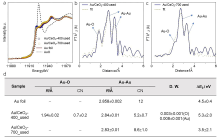

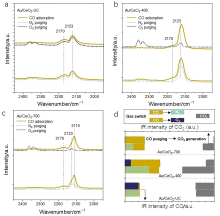

| [1] |
Haruta M.; Kobayashi T.; Sano H.; Yamada N. Chem. Lett. 1987, 16, 405.
doi: 10.1246/cl.1987.405 |
| [2] |
Hutchings G. J. J. Catal. 1985, 96, 292.
doi: 10.1016/0021-9517(85)90383-5 |
| [3] |
Jin Z.; Song Y. Y.; Fu X. P.; Song Q. S.; Jia C. J. Chin. J. Chem. 2018, 36, 639.
doi: 10.1002/cjoc.v36.7 |
| [4] |
Camellone M. F.; Fabris S. J. Am. Chem. Soc. 2009, 131, 10473.
doi: 10.1021/ja902109k |
| [5] |
Saavedra J.; Doan H. A.; Pursell C. J.; Grabow L. C.; Chandler B. D. Science 2014, 345, 1599.
doi: 10.1126/science.1256018 |
| [6] |
Liu L.; Corma A. Chem. Rev. 2018, 118, 4981.
doi: 10.1021/acs.chemrev.7b00776 |
| [7] |
Ha H.; Yoon S.; An K.; Kim H. Y. ACS Catal. 2018, 8, 11491.
doi: 10.1021/acscatal.8b03539 |
| [8] |
Lee Y.; He G.; Akey A. J.; Si R.; Flytzani-Stephanopoulos M.; Herman I. P. J. Am. Chem. Soc. 2011, 133, 12952.
doi: 10.1021/ja204479j |
| [9] |
Boccuzzi F.; Chiorino A.; Manzoli M.; Lu P.; Akita T.; Ichikawa S.; Haruta M. J. Catal. 2001, 202, 256.
doi: 10.1006/jcat.2001.3290 |
| [10] |
Guo L.-W.; Du P.-P.; Fu X.-P.; Ma C.; Zeng J.; Si R.; Huang Y.-Y.; Jia C.-J.; Zhang Y.-W.; Yan C.-H. Nat. Commun. 2016, 7, 1.
|
| [11] |
Schlexer P.; Widmann D.; Behm R. J.; Pacchioni G. ACS Catal. 2018, 8, 6513.
doi: 10.1021/acscatal.8b01751 |
| [12] |
Rodriguez J. A.; Grinter D. C.; Liu Z.; Palomino R. M.; Senanayake S. D. Chem. Soc. Rev. 2017, 46, 1824.
doi: 10.1039/C6CS00863A |
| [13] |
Ke J.; Zhu W.; Jiang Y.; Si R.; Wang Y.-J.; Li S.-C.; Jin C.; Liu H.; Song W.-G.; Yan C.-H.; Zhang Y.-W. ACS Catal. 2015, 5, 5164.
doi: 10.1021/acscatal.5b00832 |
| [14] |
Teng B.-C.; Jiang S.-Y.; Guo X.-W.; Yuan J.-H.; Luo M.-F. Acta Chim. Sinica 2009, 67, 2765. (in Chinese)
|
|
( 滕波涛, 蒋仕宇, 郭晓伟, 袁金焕, 罗孟飞, 化学学报, 2009, 67, 2765.)
|
|
| [15] |
Jiao T.; Xu X.-L.; Zhang L.; Weng Y.-Y.; Weng Y.-B.; Gao Z.-X. Acta Chim. Sinica 2021, 79, 513. (in Chinese)
doi: 10.6023/A20120562 |
|
( 焦桐, 许雪莲, 张磊, 翁幼云, 翁玉冰, 高志贤, 化学学报, 2021, 79, 513.)
|
|
| [16] |
Lin J.; Zhang L.; Ni J.; Wang R.; Wei K. Acta Chim. Sinica 2012, 70, 137. (in Chinese)
doi: 10.6023/A1107121 |
|
( 林建新, 张留明, 倪军, 王榕, 魏可镁, 化学学报, 2012, 70, 137.)
|
|
| [17] |
Spezzati G.; Benavidez A. D.; DeLaRiva A. T.; Su Y.; Hofmann J. P.; Asahina S.; Olivier E. J.; Neethling J. H.; Miller J. T.; Datye A. K.; Hensen E. J. M. Appl. Catal. B: Environ. 2019, 243, 36.
doi: 10.1016/j.apcatb.2018.10.015 |
| [18] |
Sun X.-C.; Yuan K.; Hua W.-D.; Gao Z.-R.; Zhang Q.; Yuan C.-Y.; Liu H.-C.; Zhang Y.-W. ACS Catal. 2022, 12, 11942.
doi: 10.1021/acscatal.2c03664 |
| [19] |
Si R.; Flytzani-Stephanopoulos M. Angew. Chem. Int. Ed. 2008, 47, 2884.
doi: 10.1002/(ISSN)1521-3773 |
| [20] |
Zhang S.; Lee J.; Kim D. H.; Kim T. Molecular Catalysis 2020, 482.
|
| [21] |
Yu W.-Z.; Wang W.-W.; Li S.-Q.; Fu X.-P.; Wang X.; Wu K.; Si R.; Ma C.; Jia C.-J.; Yan C.-H. J. Am. Chem. Soc. 2019, 141, 17548.
doi: 10.1021/jacs.9b05419 |
| [22] |
Manibalan G.; Murugadoss G.; Thangamuthu R.; Kumar R. M.; Jayavel R.; Kumar M. R. Materials Research Express 2019, 6, 075032.
doi: 10.1088/2053-1591/ab1634 |
| [23] |
Mihaylov M.; Knözinger H.; Hadjiivanov K.; Gates B. C. Chem. Ing. Tech. 2007, 79, 795.
doi: 10.1002/cite.v79:6 |
| [24] |
McEntee M.; Stevanovic A.; Tang W.; Neurock M.; Yates J. T. Jr. J. Am. Chem. Soc. 2015, 137, 1972.
doi: 10.1021/ja511982n |
| [25] |
Zhang S.; Li X.-S.; Chen B.; Zhu X.; Shi C.; Zhu A.-M. ACS Catal. 2014, 4, 3481.
doi: 10.1021/cs500614f |
| [26] |
El-Moemen A. A.; Kučerová G.; Behm R. J. Appl. Catal. B: Environ. 2010, 95, 57.
doi: 10.1016/j.apcatb.2009.12.009 |
| [27] |
Kang L.; Wang B.; Bing Q.; Zalibera M.; Buchel R.; Xu R.; Wang Q.; Liu Y.; Gianolio D.; Tang C. C.; Gibson E. K.; Danaie M.; Allen C.; Wu K.; Marlow S.; Sun L. D.; He Q.; Guan S.; Savitsky A.; Velasco-Velez J. J.; Callison J.; Kay C. W. M.; Pratsinis S. E.; Lubitz W.; Liu J. Y.; Wang F. R. Nat. Commun. 2020, 11, 4008.
doi: 10.1038/s41467-020-17852-8 |
| [28] |
Kim H. Y.; Lee H. M.; Henkelman G. J. Am. Chem. Soc. 2012, 134, 1560.
doi: 10.1021/ja207510v |
| [1] | Guanglong Huang, Xiao-Song Xue. Computational Study on the Mechanism of Chen’s Reagent as Trifluoromethyl Source [J]. Acta Chimica Sinica, 2024, 82(2): 132-137. |
| [2] | Guoqing Cui, Yiyang Hu, Yingjie Lou, Mingxia Zhou, Yuming Li, Yajun Wang, Guiyuan Jiang, Chunming Xu. Research Progress on the Design, Preparation and Properties of Catalysts for CO2 Hydrogenation to Alcohols [J]. Acta Chimica Sinica, 2023, 81(8): 1081-1100. |
| [3] | Yahui Jia, Chunsheng Li, Zhongzhen Xu, Wei Liu, Daowei Gao, Guozhu Chen. The SMSI of Pt-TiO2 During the Crystalline Phase Transformation and Its Effect on CO Oxidation Performance [J]. Acta Chimica Sinica, 2022, 80(9): 1289-1298. |
| [4] | Luocong Wang, Zhewei Li, Caiwei Yue, Peihuan Zhang, Ming Lei, Min Pu. Theoretical Study on the Isomerization Mechanism of Azobenzene Derivatives under Electric Field [J]. Acta Chimica Sinica, 2022, 80(6): 781-787. |
| [5] | Siming Yang, Airong Liu, Jing Liu, Zhaoli Liu, Weixian Zhang. Advance of Sulfidated Nanoscale Zero-Valent Iron: Synthesis, Properties and Environmental Application [J]. Acta Chimica Sinica, 2022, 80(11): 1536-1554. |
| [6] | Yilong Hua, Donghan Li, Tianhang Gu, Wei Wang, Ruofan Li, Jianping Yang, Wei-xian Zhang. Enrichment of Uranium from Aqueous Solutions with Nanoscale Zero-valent Iron: Surface Chemistry and Application Prospect [J]. Acta Chimica Sinica, 2021, 79(8): 1008-1022. |
| [7] | Huang Rongyi, Shen Qiong, Zhang Chao, Zhang Shaoyong, Xu Heng. Studies on the Mechanism of the Transition Metal-Catalyzed Reaction of Organonitrile with Sodium Azide [J]. Acta Chimica Sinica, 2020, 78(6): 565-571. |
| [8] | Wu Qingyuan, Qin Ruixuan, Zang Dandan, Zhang Wuyong, Wu Binghui, Zheng Nanfeng. Stabilizing Catalytic Pt-OH-Fe(III) Interfaces by Mesoporous TiO2 with Rich Surface Hydroxyl Groups [J]. Acta Chim. Sinica, 2018, 76(8): 617-621. |
| [9] | Wu Weirong, Yuan Xiaomin, Hou Hua, Wang Baoshan. Theoretical Investigations on the Mechanisms for the Reactions of Sevoflurane Radicals[(CF3)2C(·)OCH2F, (CF3)2CHOC(·)HF] with O2and the OH· Radicals Regeneration [J]. Acta Chim. Sinica, 2018, 76(10): 793-801. |
| [10] | Mu Weihua, Ma Yao, Fang Decai, Wang Rong, Zhang Haina. Computational Insights into the Diels-Alder-alike Reactions of 1-Iodo-2-Lithio-o-Carborane with Fulvenes [J]. Acta Chim. Sinica, 2018, 76(1): 55-61. |
| [11] | Huang Xiao-yue, Wang Wei, Ling Lan, Zhang Wei-xian. Heavy Metal-nZVI Reactions: the Core-shell Structure and Applications for Heavy Metal Treatment [J]. Acta Chim. Sinica, 2017, 75(6): 529-537. |
| [12] | Tang Jing, Tang Lin, Feng Haopeng, Dong Haoran, Zhang Yi, Liu Sishi, Zeng Guangming. Research Progress of Aqueous Pollutants Removal by Sulfidated Nanoscale Zero-valent Iron [J]. Acta Chim. Sinica, 2017, 75(6): 575-582. |
| [13] | Ding Shuang, Ge Qingfeng, Zhu Xinli. Research Progress in Ketonization of Biomass-derived Carboxylic Acids over Metal Oxides [J]. Acta Chim. Sinica, 2017, 75(5): 439-447. |
| [14] | Yang Zhen, Xue Yijiang, He Yuanhang. Thermal Sensitivity of CL20/DNB Co-crystal Research via Molecular Dynamics Simulations [J]. Acta Chim. Sinica, 2016, 74(7): 612-619. |
| [15] | Ma Jun, Li Rong, Ren Kuiwei, Ma Xilong, Zhu Kaili, Geng Zhiyuan. Mechanism and Spin-orbit Coupling Study for Two State Reaction Ni2+ with Cyclohexane [J]. Acta Chim. Sinica, 2015, 73(5): 431-440. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||