

HA-AuNPs/FDF for Highly Sensitive Detection of Hyaluronidase, Tumor-targeting Fluorescence Cell Imaging and Phototherapy
Received date: 2023-06-10
Online published: 2023-09-18
Supported by
Priority Academic Program Development of Jiangsu Higher Education Institutions(PAPD, YX03001); Jiangsu National Synergetic Innovation Center for Advanced Materials (SICAM), and the open research fund of State Key Laboratory of Organic Electronics and Information Displays
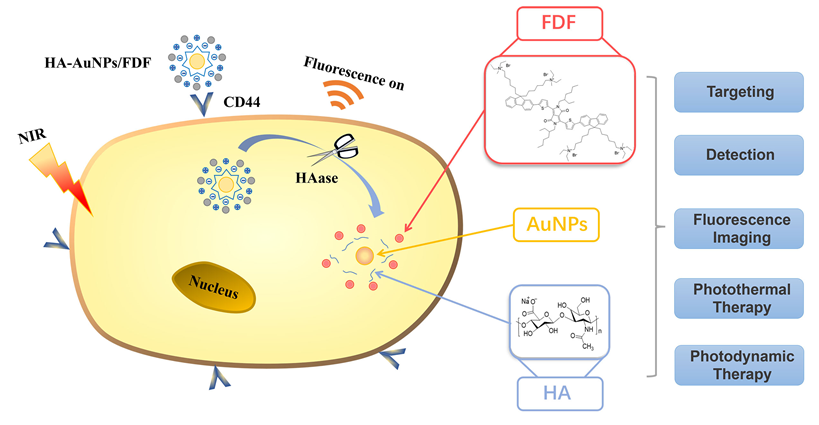
In this paper, a novel composite nano diagnostic and therapeutic agent HA-AuNPs/FDF was constructed for highly sensitive fluorescence detection of hyaluronidase (HAase), tumor-targeting fluorescence cell imaging and photodynamic/photothermal synergistic therapy. HA-AuNPs/FDF was formed by the electrostatic self-assembly of FDF, a diketopyrrolopyrrole-based conjugated small molecule, and HA-AuNPs, the gold nanoparticles functionalized with the tumor-targeting biomolecule hyaluronan (HA). FDF generated strong fluorescence under the excitation of near-infrared (NIR) light, and HA-AuNPs will quench the fluorescence of FDF through fluorescence resonance energy transfer (FRET). However, the overexpression of hyaluronidase (HAase) in tumor cells can gradually degrade HA, so FDF was released and the fluorescence of FDF was gradually recovered. Therefore, the fluorescence recovery of HA-AuNPs/FDF can be used for the rapid quantification of HAase. Experiments have shown that this method can achieve good linear response within the range of 0.25~2.25 U/mL with a detection limit of 0.04 U/mL. On this basis, after respective incubation with HA-AuNPs/FDF for 4 h, NIR fluorescence imaging was performed on human cervical cancer tumor cells (HeLa cells), HeLa cells pretreated with HA, and mouse embryonic fibroblasts (NIH-3T3 cells) to study the tumor-targeting capability of HA-AuNPs/FDF. In addition, after incubation with HA-AuNPs/FDF for 20 min, NIR fluorescence imaging was also performed on HeLa cells to detect the changes in fluorescence intensity over time and study the capability of HAase to activate fluorescence. All the results showed that HA-AuNPs/FDF was readily endocytosed by HeLa cells via the receptor CD44 and degraded by intracellular overexpressed HAase, and that it can be successfully used as a fluorescence probe activated by HAase for fluorescence cell imaging of HeLa cells. Furthermore, as FDF is a material with photothermal and photodynamic therapeutic potential, HA-AuNPs/FDF exhibited the single linear oxygen yield of 23.7%, with a photothermal conversion efficiency of 21.3% and good photothermal stability. The cytotoxicity of HA-AuNPs/FDF was measured by the 3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide (MTT) assay and live/dead cell staining experiments using calcein-acetoxymethyl ester (AM) and propidium iodide (PI) double staining kit. The results showed that the number of dead HeLa cells was significantly increased under laser irradiation as compared to that under dark conditions, confirming that HA-AuNPs/FDF can effectively inhibit the proliferation of HeLa cells by photodynamic/photothermal synergistic therapy. This system expands the ideas for achieving precise and efficient tumor diagnosis and therapy.
Yanqin Huang , Lijun Li , Shupei Yang , Rui Zhang , Xingfen Liu , Quli Fan , Wei Huang . HA-AuNPs/FDF for Highly Sensitive Detection of Hyaluronidase, Tumor-targeting Fluorescence Cell Imaging and Phototherapy[J]. Acta Chimica Sinica, 2023 , 81(12) : 1687 -1694 . DOI: 10.6023/A23060283
| [1] | Lapcik, L.; Lapcik, L.; De Smedt, S.; Demeester, J.; Chabrecek, P. Chem. Rev. 1998, 98, 2663. |
| [2] | Toole, B. P. Nat. Rev. Cancer 2004, 4, 528. |
| [3] | Xie, H. F.; Zeng, F.; Wu, S. Z. Biomacromolecules 2014, 15, 3383. |
| [4] | Lokeshwar, V. B.; Estrella, V.; Lopez, L.; Kramer, M.; Gomez, P.; Soloway, M. S.; Lokeshwar, B. L. Cancer Res. 2006, 66, 11219. |
| [5] | Kolliopoulos, C.; Bounias, D.; Bouga, H.; Kyriakopoulou, D.; Stavropoulos, M.; Vynios, D. H. J. Pharm. Biomed. Anal. 2013, 83, 299. |
| [6] | Eissa, S.; Shehata, H.; Mansour, A.; Esmat, M.; El-Ahmady, O. Med. Oncol. 2012, 29, 3345. |
| [7] | Nossier, A. I.; Eissa, S.; Ismail, M. F.; Hamdy, M. A.; Azzazy, H. M. Biosens. Bioelectron. 2014, 54, 7. |
| [8] | Martinez-Quintanilla, J.; He, D.; Wakimoto, H.; Alemany, R.; Shah, K. Mol. Ther. 2015, 23, 108. |
| [9] | Ge, M. H.; Sun, J. J.; Chen, M. L.; Tian, J. J.; Yin, H. C.; Yin, J. Anal. Bioanal. Chem. 2020, 412, 1915. |
| [10] | Diferrante, N. J. Biol. Chem. 1956, 220, 303. |
| [11] | Vercruysse, K. P.; Lauwers, A. R.; Demeester, J. M. Biochem. J. 1995, 306, 153. |
| [12] | Magalhaes, M. R.; da Silva, N. J.; Ulhoa, C. J. Toxicon 2008, 51, 1060. |
| [13] | Jayadev, C.; Rout, R.; Price, A.; Hulley, P.; Mahoney, D. J. Immunol. Methods 2012, 386, 22. |
| [14] | Carter, K. P.; Young, A. M.; Palmer, A. E. Chem. Rev. 2014, 114, 4564. |
| [15] | Chen, C.; Tian, R.; Zeng, Y.; Chu, C.; Liu, G. Bioconjug. Chem. 2020, 31, 276. |
| [16] | Cheng, D.; Han, W. Y.; Yang, K. C.; Song, Y.; Jiang, M. D.; Song, E. Q. Talanta 2014, 130, 408. |
| [17] | Huang, Y. Q.; Song, C. X.; Li, H. C.; Zhang, R.; Jiang, R. C.; Liu, X. F.; Zhang, G. W.; Fan, Q. L.; Wang, L. H.; Huang, W. ACS Appl. Mater. Interfaces 2015, 7, 21529. |
| [18] | Li, X. Q.; Zhou, Z.; Tang, Y. P.; Zhang, C. C.; Zheng, Y. H.; Gao, J. W.; Wang, Q. M. Sens. Actuators 2018, 276, 95. |
| [19] | Ge, J.; Cai, R.; Yang, L.; Zhang, L. L.; Jiang, Y.; Yang, Y.; Cui, C.; Wan, S.; Chu, X.; Tan, W. H. ACS Sustainable Chem. Eng. 2018, 6, 16555. |
| [20] | Zhang, Z.; Xu, W.; Kang, M.; Wen, H.; Guo, H.; Zhang, P.; Xi, L.; Li, K.; Wang, L.; Wang, D.; Tang, B. Z. Adv. Mater. 2020, 32, 2003210. |
| [21] | Pan, L. X.; Huang, Y. Q.; Sheng, K.; Zhang, R.; Fan, Q. L.; Huang, W. Acta Chim. Sinica 2021, 79, 1097 (in Chinese). |
| [21] | (潘立祥, 黄艳琴, 盛况, 张瑞, 范曲立, 黄维, 化学学报, 2021, 79, 1097.) |
| [22] | Gao, D.; Guo, X.; Zhang, X.; Chen, S.; Wang, Y.; Chen, T.; Huang, G.; Gao, Y.; Tian, Z.; Yang, Z. Mater. Today Bio. 2020, 5, 100035. |
| [23] | Wang, M.; Yan, D.; Wang, M.; Wu, Q.; Song, R.; Huang, Y.; Rao, J.; Wang, D.; Zhou, F.; Tang, B. Z. Adv. Funct. Mater. 2022, 32, 2205371. |
| [24] | Xu, C.; Pu, K. Chem. Soc. Rev. 2021, 50, 1111. |
| [25] | Huang, Y. Q.; Sun, L. J.; Zhang, R. ACS Appl. Bio. Mater. 2019, 2, 2421. |
| [26] | Huang, Y. Q.; Liu, K. L.; Ni, H. L.; Zhang, R.; Liu, X. F.; Fan, Q. L.; Wang, L. H.; Huang, W. ACS Appl. Polym. Mater. 2022, 4, 7739. |
| [27] | Liu, B. D.; Wang, C. J.; Qian, Y. Acta Chim. Sinica 2022, 80, 1071 (in Chinese). |
| [27] | (刘巴蒂, 王承俊, 钱鹰, 化学学报, 2022, 80, 1071.) |
| [28] | Li, Y. R.; Wang, Z. G.; Tang, Z. H. Acta Chim. Sinica 2022, 80, 291 (in Chinese). |
| [28] | (李嫣然, 王子贵, 汤朝晖, 化学学报, 2022, 80, 291.) |
| [29] | Xia, Q.; Chen, Z.; Zhou, Y.; Liu, R. Nanotheranostics 2019, 3, 156. |
| [30] | Wang, T.; Zhao, L.; Wang, K. W.; Bai, Y. F.; Feng, F. Acta Chim. Sinica 2021, 79, 600 (in Chinese). |
| [30] | (王涛, 赵璐, 王科伟, 白云峰, 冯锋, 化学学报, 2021, 79, 600.) |
| [31] | Sun, Y.; Wang, Y.; Liu, Y.; Weng, B.; Yang, H.; Xiang, Z.; Ran, J.; Wang, H.; Yang, C. ACS Appl. Mater. Interfaces 2021, 13, 53646. |
| [32] | Song, Y.; Wang, Z.; Li, L.; Shi, W.; Li, X.; Ma, H. Chem. Commun. 2014, 50, 15696. |
| [33] | Wu, Z. S.; Zhang, S. B.; Guo, M. M.; Chen, C. R.; Shen, G. L.; Yu, R. Q. Anal. Chim. Acta 2007, 584, 122. |
| [34] | Matsui, J.; Akamatsu, K.; Nishiguchi, S.; Miyoshi, D.; Nawafune, H.; Tamaki, K.; Sugimoto, N. Anal. Chem. 2004, 76, 1310. |
| [35] | Zhu, M. Q.; Wang, L. Q.; Exarhos, G. J.; Li, A. D. J. Am. Chem. Soc. 2004, 126, 2656. |
| [36] | Wu, K.; Zhao, H. H.; Sun, Z. Q.; Wang, B.; Tang, X. Y.; Dai, Y. N.; Li, M. X.; Shen, Q. M.; Zhang, H.; Fan, Q. L.; Huang, W. Theranostics 2019, 9, 7697. |
| [37] | Murphy, C. J.; Gole, A. M.; Stone, J. W.; Sisco, P. N.; Alkilany, A. M.; Goldsmith, E. C.; Baxter, S. C. Acc. Chem. Res. 2008, 41, 1721. |
| [38] | Joris, F.; Manshian, B. B.; Peynshaert, K.; De Smedt, S. C.; Braeckmans, K.; Soenen, S. J. Chem. Soc. Rev. 2013, 42, 8339. |
/
| 〈 |
|
〉 |