

Computational Study on the Mechanism of Chen’s Reagent as Trifluoromethyl Source
Received date: 2023-09-30
Online published: 2023-11-30
Supported by
Ministry of Science and Technology of China(2021YFF0701700); National Natural Science Foundation of China(22122104); National Natural Science Foundation of China(22193012); National Natural Science Foundation of China(21933004); CAS Project for Young Scientists in Basic Research(YSBR-052); CAS Project for Young Scientists in Basic Research(YSBR-095)
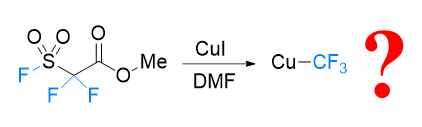
In 1989, Qing-Yun Chen’s research group at the Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences reported the development of methyl fluorosulfonyldifluoroacetate (FSO2CF2CO2Me or MFSDA) as trifluoromethylation reagent. This reagent is now known as Chen’s reagent, which is perhaps the first well-recognized and widely used trifluoromethylation reagent originate from China. Despite the widespread use of Chen’s reagent in both academia and industry, the detailed mechanism underlying the conversion of Chen’s reagent into a trifluoromethyl source has remained elusive. In this contribution, we conducted a thorough investigation into the reaction mechanism, employing density functional theory (DFT) calculations. Geometry optimizations and frequency analyses were performed using the PBE0/def2-SVP level of theory. To ensure accurate electronic energy calculations, single-point energy calculations were conducted at the ωB97X-D/def2-TZVPP level of theory. The solvent effects were considered using the solvation model density (SMD) model during both geometry optimizations and single-point energy calculations. Furthermore, Gibbs free energies were corrected with GoodVibes, employing Truhlar et al.’s quasi-harmonic treatment by setting all positive frequencies less than 100 to 100 cm–1. Concentration corrections were applied from 1 atm to 1 mol/L. Our calculations reveal the detailed mechanism governing the generation of copper(I) trifluoromethyl from Chen’s reagent in the presence of a CuI catalyst. An in-depth understanding of such mechanistic details would be helpful for future development of new reaction and application with Chen’s reagent.
Guanglong Huang , Xiao-Song Xue . Computational Study on the Mechanism of Chen’s Reagent as Trifluoromethyl Source[J]. Acta Chimica Sinica, 2024 , 82(2) : 132 -137 . DOI: 10.6023/A23090434
| [1] | (a) Chen, Q.-Y.; Zhu, S. Z. Sci. China Chem. B 1986, 6, 561. (in Chinese) |
| [1] | (陈庆云, 朱仕正, 中国科学(B辑), 1986, 6, 561.) |
| [1] | (b) Chen, Q.-Y.; Wu, S.-W. J. Chem. Soc., Chem. Commun. 1989, 705. |
| [2] | (a) Jiang, X. K. Acta Chim. Sinica 1957, 5, 330. (in Chinese) |
| [2] | (蔣錫夔, 化学学报, 1957, 5, 330.) |
| [2] | (b) Olah, G. A.; Iyer, P. S.; Prakash, G. K. S. Synthesis 1986, 513. |
| [2] | (c) England, D. C. US 2852554, 1958. |
| [2] | (d) England, D. C.; Dietrich, M. A.; Lindsey, R. V. J. Am. Chem. Soc. 1960, 82, 6181. |
| [2] | (e) Dmitriev, M. A.; Sokol'skii, G. A.; Knunyants, I. L. Bull. Acad. Sci. USSR, Div. Chem. Sci. 1960, 9, 792. |
| [2] | (f) Dmitriev, M. A.; Sokol'skii, G. A.; Knunyants, I. L. Bull. Acad. Sci. USSR, Div. Chem. Sci. 1960, 9, 966. |
| [2] | (g) Zhao, G.; Wu, H.; Xiao, Z.; Chen, Q.-Y.; Liu, C. RSC Adv. 2016, 6, 50250. |
| [2] | (h) Zhang, C.-P.; Chen, Q.-Y; Guo, Y.; Xiao, J.-C.; Gu, Y.-C. Coord. Chem. Rev. 2014, 261, 28. |
| [3] | Chen, Q.-Y.; Yang, G.-Y.; Wu, S.-W. J. Fluorine Chem. 1991, 55, 291. |
| [4] | Zhao, S.; Guo, Y.; Han, E.-J.; Luo, J.; Liu, H.-M.; Liu, C.; Xie, W.; Zhang, W.; Wang, M. Org. Chem. Front. 2018, 5, 1143. |
| [5] | Chen, Q.-Y. J. Fluorine Chem. 1995, 72, 241. |
| [6] | (a) Eusterwiemann, S.; Martinez, H.; Dolbier, W. R. J. Org. Chem. 2012, 77, 5461. |
| [6] | (b) Thomoson, C. S.; Martinez, H.; Dolbier, W. R. J. Fluorine Chem. 2013, 150, 53. |
| [6] | (c) Yu, W.; Xu, X.-H.; Qing, F.-L. Org. Lett. 2016, 18, 5130. |
| [6] | (d) Zhao, G.; Wu, H.; Xiao, Z.; Chen, Q.-Y.; Liu, C. RSC Adv. 2016, 6, 50250. |
| [6] | (e) Liu, Y.; Wu, H.; Guo, Y.; Xiao, J.-C.; Chen, Q.-Y.; Liu, C. Angew. Chem., Int. Ed. 2017, 56, 15432. |
| [7] | (a) Tomashenko, O. A.; Grushin, V. V. Chem. Rev. 2011, 111, 4475. |
| [7] | (b) Clarke, S. L.; McGlacken, G. P. Chem. Eur. J. 2016, 22, 1. |
| [7] | (c) Xie, Q.; Hu, J. Chin. J. Chem. 2020, 38, 202. |
| [7] | (d) Chen, Q. Chin. J. Org. Chem. 2001, 21, 805. (in Chinese) |
| [7] | (陈庆云, 有机化学, 2001, 21, 805.) |
| [8] | (a) Wiemers, D. M.; Burton, D. J. J. Am. Chem. Soc. 1986, 108, 832. |
| [8] | (b) Dubinina, G. G.; Furutachi, H.; Vicic, D. A. J. Am. Chem. Soc. 2008, 130, 8600. |
| [8] | (c) Morimoto, H.; Tsubogo, T.; Litvinas, N. D.; Hartwig, J. F. Angew. Chem., Int. Ed. 2011, 50, 3793. |
| [8] | (d) Litvinas, N. D.; Fier, P. S.; Hartwig, J. F. Angew. Chem., Int. Ed. 2012, 51, 536. |
| [8] | (e) Ni, C.; Hu, J. Synthesis 2014, 46, 842. |
| [8] | (f) Konovalov, A. I.; Lishchynskyi, A.; Grushin, V. V. J. Am. Chem. Soc. 2014, 136, 13410. |
| [8] | (g) de Salinas, S. M.; Mudarra, á. L.; Odena, C.; Belmonte, M. M.; Benet-Buchholz, J.; Maseras, F.; Pérez-Temprano, M. H. Chem. Eur. J. 2019, 25, 9390. |
| [8] | (h) Yu, W.; Ouyang, Y.; Xu, X.-H.; Qing, F.-L. Chin. J. Chem. 2018, 36, 1024. |
| [8] | (i) Zhang, W.; Lin, J.-H.; Wu, W.; Cao, Y.-C.; Xiao, J.-C. Chin. J. Chem. 2020, 38, 169. |
| [9] | Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Petersson, G. A.; Nakatsuji, H.; Li, X.; Caricato, M.; Marenich, A. V.; Bloino, J.; Janesko, B. G.; Gomperts, R.; Mennucci, B.; Hratchian, H. P.; Ortiz, J. V.; Izmaylov, A. F.; Sonnenberg, J. L.; Williams-Young, D.; Ding, F.; Lipparini, F.; Egidi, F.; Goings, J.; Peng, B.; Petrone, A.; Henderson, T.; Ranasinghe, D.; Zakrzewski, V. G.; Gao, J.; Rega, N.; Zheng, G.; Liang, W.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Throssell, K.; Montgomery Jr., J. A.; Peralta, J. E.; Ogliaro, F.; Bearpark, M. J.; Heyd, J. J.; Brothers, E. N.; Kudin, K. N.; Staroverov, V. N.; Keith, T. A.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A. P.; Burant, J. C.; Iyengar, S. S.; Tomasi, J.; Cossi, M.; Millam, J. M.; Klene, M.; Adamo, C.; Cammi, R.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Farkas, O.; Foresman, J. B.; Fox, D. J. Gaussian 16, Revision A.03, Gaussian, Inc., Wallingford CT, 2016. |
| [10] | Marenich, A. V.; Cramer, C. J.; Truhlar, D. G. J. Phys. Chem. B 2009, 113, 6378. |
| [11] | (a) Zhao, Y.; Truhlar, D. G. J. Chem. Phys. 2006, 125, 194101. |
| [11] | (b) Quintal, M. M.; Karton, A.; Iron, M. A.; Boese, A. D.; Martin, J. M. L. J. Phys. Chem. A 2006, 110, 709. |
| [12] | Weigend, F.; Ahlrichs, R. Phys. Chem. Chem. Phys. 2005, 7, 3297. |
| [13] | Chai, J.-D.; Head-Gordon, M. Phys. Chem. Chem. Phys. 2008, 10, 6615. |
| [14] | Weigend, F. Phys. Chem. Chem. Phys. 2006, 8, 1057. |
| [15] | Funes-Ardoiz, I.; Paton, R. S., GoodVibes v2. 0.2, 2016, (http://doi.org/10.5281/zenodo.595246). |
| [16] | Legault, C. Y., CYLview, 1.0b, Université de Sherbrooke, 2009. (http://www.cylview.org). |
/
| 〈 |
|
〉 |