

The Regulation of Stereoselectivity in Radical Polymerization★
Received date: 2023-10-10
Online published: 2024-01-05
Supported by
Youth Innovation Promotion Association CAS(2020259); National Natural Science Foundation of China(22271304); Shanghai Rising-Star Program(21QA1411200); Shanghai Natural Science Foundation(23ZR1476300)
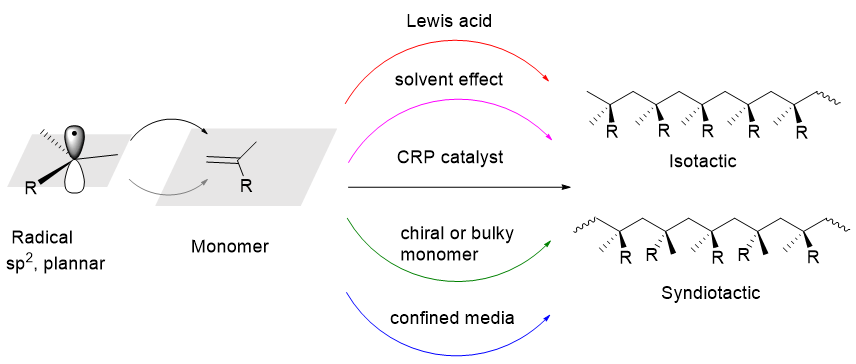
The physical and chemical properties of polymer strongly depend on the structures of the polymer chain. The structure studies range from the structure of repeat units and their mole fractions to more detailed microstructures, such as molecular weight and its distribution, the sequence of monomer units (block, gradient, alternating, graft, etc.), topology (stars, combs, networks, brushes, etc.), the end-functionalities and tacticity. Among them, the regulation of the polymer tacticity is greatly significant. For example, the stereospecific coordination polymerization of propylene has shown great commercial value. The control of macromolecular structure is difficult for free radical polymerization, although it is one of the most widely used polymerization techniques in the production of polymer materials. The development of reversible deactivated radical polymerization (RDRP) has significantly improved the control over the molecular weight of polymer. However, the regulation of stereoselectivity is extremely challenging for all forms of radical polymerization. One of the reasons is because a terminal carbon of propagating radical takes essentially a neutral sp2 planar-like structure without counter species in contrast to an anionic sp3 pyramidal or cationic sp2 planar-like structure with counter ion or chiral catalyst site. This brings about a non-stereospecific radical propagation, and results in the energy difference between two enantiomers of an active radical species is small and the energy barrier between the enantiomers is low compared to the thermal energy at the polymerization temperature. In this review, the regulation of stereoselectivity in free radical polymerization are comprehensively summarized, evaluated, and prospected from the perspective of regulation strategies, including polymerization in restricted environment, using monomer containing chiral or bulky substituents, solvent (hydrogen bond) effect, the addition of Lewis acid and catalyst or ligand effect. Although these strategies have achieved some preliminary progress, there are still some problems in general, such as limited monomer scope, high solvent cost, complex reaction system, high Lewis acid loading, low catalytic efficiency and insignificant regulatory effect. The use of controlled radical polymerization catalysts to regulate stereoselectivity is of great potential. In the future, the studies on the radical asymmetric catalysis of small molecule can be used for reference to carefully design the structure of the catalyst and optimize the reaction conditions. In this way, the distance between the catalytic center and the terminal radical of the polymer chain is narrowed, and a confined space environment is created to enhance the stereochemical influence of the catalyst structure on the radical addition polymerization process.
Key words: radical polymerization; stereoselectivity; mechanism; tactic polymer; catalyst
Yaning Li , Xiaoyan Wang , Yong Tang . The Regulation of Stereoselectivity in Radical Polymerization★[J]. Acta Chimica Sinica, 2024 , 82(2) : 213 -225 . DOI: 10.6023/A23100445
| [1] | (a) Matyaszewski, K.; Davis, T. P. Handbook of Radical Polymerization, Wiley-Interscience, Hoboken, 2002. |
| [1] | (b) Kamigaito, M.; Ando, T.; Sawamoto, M. Chem. Rev. 2001, 101, 3689. |
| [1] | (c) Matyjaszew- ski, K.; Xia, J. Chem. Rev. 2001, 101, 2921. |
| [1] | (d) Li, H.-M.; Wang, J.; Ni, Y.-Z.; Zhou, Y.-F.; Yan, D.-Y. Acta Chim. Sinica 2016, 74, 415. (in Chinese) |
| [1] | (李惠梅, 王洁, 倪云洲, 周永丰, 颜德岳, 化学学报, 2016, 74, 415.) |
| [1] | (e) Li, L.-L.; Xiang, Y.-Y.; Liu, H.; Ma, S.-H.; Li, B.; Ma, Z.-Q.; Wei, Q.-B.; Yu, B.; Zhou, F. Acta Chim. Sinica 2021, 79, 353. (in Chinese) |
| [1] | (李乐乐, 向阳阳, 刘欢, 麻拴红, 李斌, 马正峰, 魏强兵, 于波, 周峰, 化学学报, 2021, 79, 353.) |
| [1] | (f) Lin, D.-N.; Zhang, L.; Tan, J.-B. Acta Polym. Sinica 2023, 54, 761. (in Chinese) |
| [1] | (林冬妮, 张力, 谭剑波, 高分子学报, 2023, 54, 761.) |
| [2] | (a) Teator, A. J.; Varner, T. P.; Knutson, P. C.; Sorensen, C. C.; Leibfarth, F. A. ACS Macro Lett. 2020, 9, 1638. |
| [2] | (b) Li, Y.; Wang, X.-Y.; Tang, Y. Acta Chim. Sinica 2021, 79, 1320. (in Chinese) |
| [2] | (李勇, 王晓艳, 唐勇, 化学学报, 2021, 79, 1320.) |
| [2] | (c) Jiang, Y.; Zhang, Z.; Li, S.; Cui, D. Angew. Chem., Int. Ed. 2022, 61, e202112966. |
| [2] | (d) Mariott, W. R.; Chen, E. Y.-X. J. Am. Chem. Soc 2002, 124, 5612. |
| [2] | (e) Zhang, X.; Yang, Z.; Jiang, Y.; Liao, S. J. Am. Chem. Soc 2021, 144, 679. |
| [2] | (f) Zhu, H.; Jiang, Y.; Yang, Z.; Zhang, X.; Liao, S. Giant 2023, 14, 100151. |
| [3] | (a) Sciannamea, V.; Jerome, R.; Detrembleur, C. Chem. Rev. 2008, 108, 1104. |
| [3] | (b) Fairbanks, B. D.; Gunatillake, P. A.; Meagher, L. Adv. Drug Delivery Rev. 2015, 91, 141. |
| [4] | Kamigaito, M.; Satoh, K. Macromolecules 2008, 41, 269. |
| [5] | Satoh, K.; Kamigaito, M. Chem. Rev. 2009, 109, 5120. |
| [6] | Wegner, G. Z. Naturforsch., B 1969, 24, 824. |
| [7] | Matsumoto, A.; Matumura, T.; Aoki, S. J. Chem. Soc., Chem. Commun. 1994, 1389. |
| [8] | Hema, K.; Ravi, A.; Raju, C.; Pathan, J. R.; Rai, R.; Sureshan, K. M. Chem. Soc. Rev. 2021, 50, 4062. |
| [9] | Grisenthwaite, R. J.; Hunter, R. F. Chem. Ind. 1959, 433. |
| [10] | Minanawa, M.; Yamada, H.; Yamaguchi, K.; Yoshii, F. Macromolecules 1992, 25, 503. |
| [11] | Farina, M.; Audisio, G.; Natta, G. J. Am. Chem. Soc. 1967, 89, 5071. |
| [12] | Bartels, T.; Tan, Y. Y.; Challa, G. J. Polym. Sci., Polym. Chem. Ed. 1977, 15, 341 |
| [13] | (a) Narumi, A.; Baba, H.; Akabane, T.; Saito, Y.; Ohno, S.; Togashi, D.; Enomoto, K.; Kikuchi, M.; Haba, O.; Kawaguchi, S. Macromolecules 2015, 48, 3395. |
| [13] | (b) Hwang, J. H.; Lee, H. C.; Antonietti, M.; Schmidt, B. V. K. J. Polym. Chem. 2017, 8, 6204. |
| [14] | Nakano, T.; Okamoto, Y. Chem. Rev. 2001, 101, 4013. |
| [15] | Porter, N. A.; Allen, T. R.; Breyer, R. A. J. Am. Chem. Soc. 1992, 114, 7676. |
| [16] | (a) Wu, W. X.; Mcphail, A. T.; Porter, N. A. J. Org. Chem. 1994, 59, 1302. |
| [16] | (b) Tanaka, H.; Niwa, M. Polymer 2008, 49, 3693. |
| [16] | (c) Nakano, H.; Kinjo, N.; Hidaka, Y.; Okamoto, Y. Polym. J. 1999, 31, 464. |
| [16] | (d) Nakano, T.; Sogah, D. Y. J. Am. Chem. Soc. 1995, 117, 534. |
| [16] | (e) Fujita, T.; Yamago, S. Chem. Eur. J. 2015, 21, 18547. |
| [17] | Tanaka, H.; Maki, K.; Matsubara, Y. J. Polym. Sci., Part A: Polym. Chem. 2018, 56, 184. |
| [18] | (a) Lando, J. B.; Litt, M.; Kumar, N. G.; Shimko, T. M. J. Polym. Sci., Part C: Symp. 1974, 44, 203. |
| [18] | (b) Yamada, K; Nakano, T.; Okamoto, Y. Macromolecules 1998, 31, 7598. |
| [18] | (c) Isobe, Y.; Yamada, K.; Nakano, T.; Okamoto, Y. J. Polym, Sci., Part A: Polym. Chem. 2000, 38, 4693. |
| [18] | (d) Zhang, J.; Liu, W.; Nakamo, T.; Okamoto, Y. Polym. J. 2000, 32, 694. |
| [18] | (e) De?irmenci, I.; Eren, ?.; Aviyente, V.; De Sterck, B.; Hemelsoet, K.; Van Speybroeck, V.; Waroquier, M. Macromolecules 2010, 43, 5602. |
| [18] | (f) Miura, Y.; Satoh, A.; Nishizawa, Y.; Okamoto, Y.; Kakuchi, T. Macromolecules 2005, 38, 1041. |
| [18] | (g) Shim, S.-H.; Ham, M.-K.; Huh, J.; Kwon, Y.-K.; Kwark, Y.-J. Polym. Chem. 2013, 4, 5449. |
| [18] | (h) Li, N.; Ding, D.; Pan, X.; Zhang, Z.; Zhu, J.; Boyer, C.; Zhu, X. Polym. Chem. 2017, 8, 6024. |
| [18] | (i) Wan, D.; Satoh, K.; Kamigaito, M. Macromolecules 2006, 39, 6882. |
| [18] | (j) Tao, Y.; Satoh, K.; Kamigaito, M. Macromol. Rapid Commun. 2010, 32, 226. |
| [19] | Matsumoto, A.; Nakamura, S. J. Appl. Polym. Sci. 1999, 74, 290. |
| [20] | Habaue, S.; Baraki, H.; Okamoto, Y. Polym. J. 2000, 32, 1017. |
| [21] | Isobe, Y.; Nakano, T.; Okamoto, Y. J. Polym. Sci., Part A: Polym. Chem. 2001, 39, 1463. |
| [22] | Noble, B. N.; Smith, L. M.; Coote, M. L. Polym. Chem. 2014, 5, 4974. |
| [23] | Schaubach, S.; Wang, X.-Y.; Li, J.-F.; Sun, X.-L.; Wang, S. R.; Tang, Y. Polym. Chem. 2018, 9, 4711. |
| [24] | Samal, S.; Thompson, B. C. ACS Macro Lett. 2018, 7, 1161. |
| [25] | Isobe, Y.; Fujioka, D.; Habaue, S.; Okamoto, Y. J. Am. Chem. Soc. 2001, 123, 7180. |
| [26] | Lutz, J. F.; Neugebauer, D.; Matyjaszewski, K. J. Am. Chem. Soc. 2003, 125, 6986. |
| [27] | Shanmugam, S.; Boyer, C. J. Am. Chem. Soc. 2015, 137, 9988. |
| [28] | Sun, Y.; Fu, L. Y.; Olszewski, M.; Matyjaszewski, K. Macromol. Rapid Commun. 2019, 40, 1800877. |
| [29] | (a) Imamura, Y.; Fujita, T.; Kobayashi, Y.; Yamago, S. Polym Chem. 2020, 11, 7042. |
| [29] | (b) Park, B.; Imamura, Y.; Yamago, S. Polym. J. 2021, 53, 515. |
| [30] | (a) Jiang, X.; Xiong, W.; Deng, S.; Lu, F.-D.; Jia, Y.; Yang, Q.; Xue, L.-Y.; Qi, X.; Tunge, J. A.; Lu, L.-Q.; Xiao, W.-J. Nat. Catal. 2022, 5, 788. |
| [30] | (b) Liu, X.; Liu, B.; Liu, Q. Angew. Chem., Int. Ed. 2020, 59, 6750. |
| [30] | (c) Li, L.-J.; He, Y.; Yang, Y.; Guo, J.; Lu, Z.; Wang, C.; Zhu, S.; Zhu, S.-F. CCS Chem. 2023, DOI: 10.31635/ccschem.023.202303412. |
| [30] | (d) Wu, Z. Q.; Peng, C. H.; Fu, X. F. Polym. Chem. 2020, 11, 4387. |
| [31] | Zhang, X.; Lin, F.; Cao, M.; Zhong, M. Nat. Synth. 2023, https://doi.org/10.1038/s44160-023-00311-9. |
| [32] | Haddleton, D. M.; Duncalf, D. J.; Kululj, D.; Heming, A. M.; Shooter, A. J.; Clark, A. J. J. Mater. Chem. 1998, 8, 1525. |
| [33] | Yu, B.; Ruckenstein, E. J. Polym. Sci., Part A: Polym. Chem. 1999, 37, 4191. |
| [34] | Johnson, R. M.; Ng, C.; Samson, C. C. M.; Fraser, C. L. Macromolecules 2000, 33, 8618. |
| [35] | Stoffelbach, F.; Richard, P.; Poli, R.; Jenny, T.; Savary, C. Inorg. Chim. Acta 2006, 359, 4447. |
| [36] | Kameyama, M.; Kamigata, N.; Kobayashi, M. J. Org. Chem. 1987, 52, 3312. |
| [37] | Iizuka, Y.; Li, Z.; Satoh, K.; Kamigaito, M.; Okamoto, Y.; Ito, J.; Nishiyama, H. Eur. J. Org. Chem. 2007, 782. |
| [38] | (a) Zhou, J.; Tang, Y. Chem. Soc. Rev. 2005, 34, 664. |
| [38] | (b) Liao, S.-H.; Sun, X.-L.; Tang, Y. Acc. Chem. Res. 2014, 47, 2260. |
| [38] | (c) Sun, X.-L; Tang, Y. Acta Polym. Sinica 2017, 7, 1019. (in Chinese) |
| [38] | (孙秀丽, 唐勇, 高分子学报, 2017, 7, 1019.) |
| [39] | (a) Wang, X.-Y.; Sun, X.-L.; Wang, F.; Tang, Y. ACS Catal. 2017, 7, 4692. |
| [39] | (b) Chen, Z.-H.; Wang, X.-Y.; Sun, X.-L.; Li, J.-F.; Zhu, B.-H.; Tang, Y. Macromolecules 2019, 52, 9792. |
| [39] | (c) Chen, Z.-H.; Ma, Y.; Wang, X.-Y.; Sun, X.-L.; Li, J.-F.; Zhu, B.-H.; Tang, Y. ACS Catal. 2020, 10, 14127. |
| [39] | (d) Wang, X.-Y.; Sun, X.-L.; Chen, Z.-H.; Wang, F.; Wang, R. S.; Tang, Y. Polym. Chem. 2018, 9, 4309. |
| [39] | (e) Wang, X.-Y.; Chen, Z.-H.; Sun, X.-L.; Tang, Y. Polymer 2019, 178, 121630. |
| [39] | (f) Ma, Y.; Yang, H.-M.; Chen, Z.-H.; Li, Y.-N.; Li, J.-F.; Sun, X.-L.; Wang, X.-Y.; Tang, Y. Polym. Chem. 2021, 12, 6606. |
/
| 〈 |
|
〉 |