

A Chiral Polymer of Intrinsic Microporosity for Fluorescent Recognition
Received date: 2024-06-11
Online published: 2024-07-18
Supported by
National Natural Science Foundation of China(22078174); University-Industry Collaborative Education Program(231106429150016); Jiangsu Future Membrane Technology Innovation Center(KF2024004)
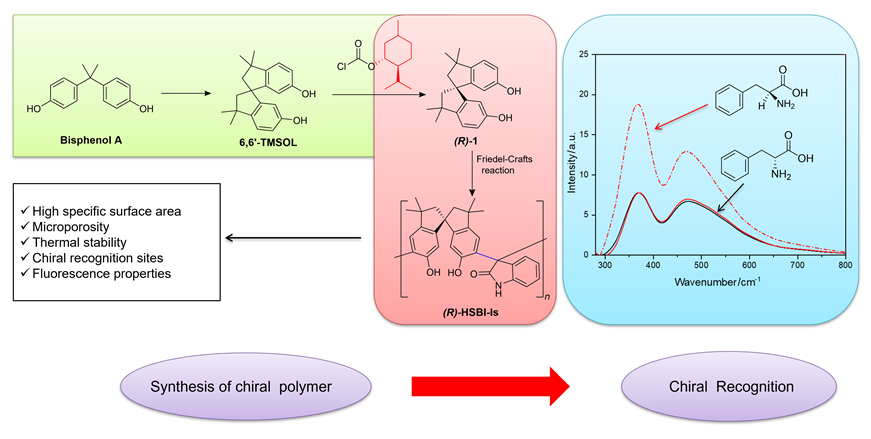
Chirality, a ubiquitous phenomenon in nature, profoundly influences material properties. The precise synthesis of chiral compounds and enantiomer recognition techniques hold promise as pivotal advancements in the realms of medicine, informatics, and materials science, yet remain a formidable challenge. In this paper, we prepared a monochiral self-forming microporous polymers ((R)-HSBI-Is) through direct-synthesis method, and applied it for chiral recognition of phenylalanine. First, commencing with bisphenol A, racematic 3,3,3',3'-tetramethyl-1,1'-spirodine-7,7'-diol (6,6'-TMSOL) was synthesized, and form transitional complex derivates through adding chiral reagents (L-menthyl chloroformate). After three times recrystallization under -18 ℃, the diastereoisomers of the complex derivates were successfully separated due to their different solubility. After hydrolysis reaction, the auxiliary groups were removed and chiral monomer was synthesized. This study achieved the artful synthesis of pure (R)-3,3,3',3'-tetramethyl-1,1'-spirodine-7,7'-diol ((R)-6,6'-TMSOL). Subsequently, chiral polymer of intrinsic microporosity ((R)-HSBI-Is) were crafted through Friedel-Crafts reaction with isatin molecules. And we delved the mechanism of synthesizing chiral polymers. These polymers were comprehensively evaluated using techniques such as nuclear magnetic resonance, infrared spectroscopy, UV spectroscopy, specific surface area and pore size analysis, and thermogravimetric analysis. Our findings reveal that the polymer boasts a high specific surface area and robust fluorescence. It also shows a great performance in thermal stability. The fluorescence experiments on chiral molecules (phenylalanine, tryptophan, 1,1'-binaphthyl-2,2'-diol (BINOL), 1,1'-spirobiindane (SPINOL) and 1-phenylethanol) were carried out to testify the recognition performance of chiral polymer. The results proved that the polymer exhibits a remarkable selective fluorescence enhancement towards L-phenylalanine. Specifically, the L- and D-configuration selectivity achieved is 34.9, positioning it as an exceptional chiral recognition agent for L-phenylalanine.
Yu Wang , Cong Yu , Yanfang Xia , Lan Lin , Qiang Chen , Xinbo Wang . A Chiral Polymer of Intrinsic Microporosity for Fluorescent Recognition[J]. Acta Chimica Sinica, 2024 , 82(8) : 914 -918 . DOI: 10.6023/A24060191
| [1] | Youatt, J. B.; Brown, R. D. Science 1981, 212, 1145. |
| [2] | Maier, N. M.; Franco, P.; Lindner, W. J. Chromatogr. A 2001, 906, 3. |
| [3] | Amako, Y.; Woo, C. M. Nat. Chem. 2019, 11, 1080. |
| [4] | Teng, Y.; Gu, C.-L.; Chen, Z.-H.; Jiang, H.; Xiong, Y.; Liu, D.; Xiao, D. Chirality 2022, 34, 1094. |
| [5] | D'orazio, G.; Fanali, C.; Asensio-Ramos, M.; Fanali, S. TrAC, Trends Anal. Chem. 2017, 96, 151. |
| [6] | Abad-Gil, L.; Marina, M. L. J. Chromatogr. Open 2023, 4, 100099. |
| [7] | Scriba, G. K. E. J. Chromatogr. A 2016, 1467, 56. |
| [8] | (a) Zou, J.; Zhao, G.-Q.; Zhao, G.-L.; Yu, J.-G. Coord. Chem. Rev. 2022, 471, 214732. |
| [8] | (b) Niu, X.-H.; Yang, X.; Li, H.-X.; Liu, J.; Liu, Z.-Y.; Wang, K.-J. Microchim. Acta 2020, 187, 676. |
| [9] | (a) Lu, S.-Y.; Liang, Y.; Wang, L.-M.; Huang, S.; Xiao, Q. Spectrosc. Spectr. Anal. 2016, 36(S1), 10 (in Chinese). |
| [9] | (卢双燕, 梁瑜, 王鲁敏, 黄珊, 肖琦, 光谱学与光谱分析, 2016, 36(S1), 10.) |
| [9] | (b) Tian, X.-M.; Lin, Y.-Q.; Zhu, H.; Huang, C.; Zhu, B.-X. Acta Chim. Sinica 2023, 81, 20 (in Chinese). |
| [9] | (田小茂, 林悦群, 朱菡, 黄超, 朱必学, 化学学报, 2023, 81, 20.) |
| [10] | (a) Zhao, Y.; Liu, H.-L.; Sun, B.-G. Sens. Actuators, B 2022, 354, 131253. |
| [10] | (b) Chen, J.; Wang, Y.-T.; Yu, Y.-L.; Wang, J.-H.; Liu, J.-W.; Ihara, H.; Qiu, H.-D. Exploration 2023, 3, 20220144. |
| [11] | Chen, A.-L.; Zhong, Y.-J.; Yin, X.-H.; Li, R.-J.; Deng, Q.-F.; Yang, R. Sens. Actuators, B 2023, 393, 134262. |
| [12] | Anum, D.; Bwambok, D. K. J. Mol. Liq. 2023, 390, 123141. |
| [13] | Zhao, Y.-W.; Guo, L.-E.; Zhang, F.-Q.; Yao, J.; Zhang, X.-M. ACS Appl. Mater. Interfaces 2021, 13, 20821. |
| [14] | (a) Lu, Z.-Y.; Lu, X.-T.; Zhong, Y.-H.; Hu, Y.-F.; Li, G.-K.; Zhang, R.-K. Anal. Chim. Acta 2019, 1050, 146. |
| [14] | (b) Chen, L.-Y.; Tan, M.-X.; Jin, J.-N.; Zhang, Z.-B.; Huang, F.-H.; Li, S.-J.; Li, Y.-X. Chinese J. Org. Chem. 2024, 44, DOI: 10.6023/cjoc202401011 (in Chinese). |
| [14] | (陈璐怡, 谭梦霞, 金迦南, 张子彬, 黄飞鹤, 李世军, 李云霞, 有机化学, 2024, 44, DOI: 10.6023/cjoc202401011.) |
| [15] | Jafari, M.; Tashkhourian, J.; Absalan, G. Spectrochim. Acta, Part A 2017, 185, 77. |
| [16] | Wang, X.-B.; Guo, H.; Yu, C.; Jing, Y.-J.; Han, Z.-B.; Ma, X.-H.; Yang, C.-C.; Liu, M.-H.; Zhai, D.; Zheng, D.-Y.; Pan, Y.-P.; Li, X.-X.; Ding, K.-L. Macromolecules 2021, 54, 11180. |
| [17] | Weng, X.-L.; Baez, J. E.; Khiterer, M.; Hoe, M. Y.; Bao, Z.-B.; Shea, K. J. Angew. Chem. Int. Ed. 2015, 54, 11214. |
| [18] | Zhang, Q.-P.; Wang, Z.; Zhang, Z.-W.; Zhai, T.-L.; Chen, J.-J.; Ma, H.; Tan, B.-E.; Zhang, C. Angew. Chem. Int. Ed. 2021, 60, 12781. |
| [19] | Feizi, F.; Shamsipur, M.; Gholivand, M. B.; Taherpour, A.; Barati, A.; Shamsipur, H.; Mohajerani, E.; Budd, P. J. Mater. Chem. C 2020, 8, 13827. |
| [20] | Chen, W.-F.; Lin, H.-Y.; Dai, S.-A. Org. Lett. 2004, 6, 2341. |
| [21] | Zhu, Z.-Y.; Dong, H.; Li, K.-H.; Li, Q.-X.; Li, J.-X.; Ma, X.-H. Sep. Purif. Technol. 2021, 262, 118313. |
| [22] | Luo, H.-H.; Yang, F.; Li, C.; Zhong, Y.-F.; Cheng, C.; Wang, S.-L.; Jin, S.-B. Chem. Eng. J. 2023, 476, 146611. |
| [23] | (a) Xu, C.-M.; Qi, Y.-S.; Yang, X.-S.; Li, X.-F.; Li, Z.-P.; Bai, L. Org. Lett. 2021, 23, 2890. |
| [23] | (b) Lin, X.-F.; Yao, L.-X.; Chang, S.-R.; Wang, L. CN108659041A, 2017 [Chem. Abstr. 2017, 169, 526105] (in Chinese). |
| [23] | (林旭锋, 姚林曦, 常时瑞, 王雷, CN108659041A, 2017, [Chem. Abstr. 2017, 169, 526105].) |
/
| 〈 |
|
〉 |