

Synthesis and Characterization of Chiral Luminescent Materials Based on Binaphthol Scaffolds
Received date: 2024-10-07
Online published: 2024-12-02
Supported by
Natural Science Foundation of Beijing(2232024); Experiment Based Project of BIT(2023BITSYB09)
The study of chiroptoelectronic materials that combine stereochemistry and luminescent properties represents one of the frontiers in areas of chirality science. Of particular interest is the access of organic chiral small molecules that can really show well-defined molecular structures and potentially unique structure-function relationships. In this article, we designed and synthesized three new examples of chiral luminescent compounds (M1, M2 and M3), where the axial chirality is derived from the incorporated binaphthyl groups. All these molecules feature an electron donor-acceptor (D-A) charge-transfer (CT) system, and they were prepared by palladium-catalyzed C—N coupling reactions between the electron-donating carbazole or biarylamines and binaphthyls that were further reacted with 2,3,5,6-tetrafluoro-1,4-dicyanoben- zene as a strong electron-accepting moiety. The structures of M1, M2 and M3 have been verified by 1H, 13C NMR and high-resolution mass spectrometry. Their electronic properties were characterized by UV-vis absorption and emission spectra as well as density functional theory (DFT) calculations. The electrochemistry has been performed by cyclic and differential pulse voltammetry, which showed reversible oxidations on the electron donor sites. The enantiomers of these chiral molecules synthesized here were optically resolved through chiral high performance liquid chromatography (HPLC) with a high value of enantiomeric excess (ee>98%), and the chiroptical properties in the ground and excited states were investigated by circular dichroism (CD) and circularly polarized luminescence (CPL) spectra, respectively. Our computations disclosed a small singlet-triplet energy gap (∆ES-T), and the experimental results gave rise to an enhanced charge-transfer emission behavior with increasing temperature, likely indicating the potential applications in thermally activated delayed fluorescent (TADF) materials. Solvatochromic emissions have been observed for all the molecules as changes in solvent polarity due to the intramolecular charge-transfer effects. Emission color changes were also reversibly induced in solutions in response to changes in temperature, as a result of stabilization of CT states by the temperature-dependent polarity of solvents.
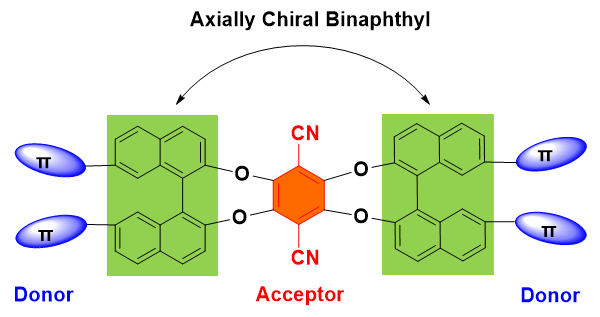
Key words: chiroptics; binaphthol; electron donor-acceptor; triarylamine; carbazole
Niu Zhang , Shuran Han , Hongwei Ma , Pangkuan Chen . Synthesis and Characterization of Chiral Luminescent Materials Based on Binaphthol Scaffolds[J]. Acta Chimica Sinica, 2025 , 83(1) : 10 -16 . DOI: 10.6023/A24100296
| [1] | (a) Yang, X. F.; Gao, X. Q.; Zheng, Y. X.; Kuang, H.; Chen, C. F.; Liu, M. H.; Duan, P. F.; Tang, Z. Y. CCS Chem. 2023, 5, 2760. |
| [1] | (b) Xue, Y. T.; Shi, Y. F.; Chen, P. K. Adv. Opt. Mater. 2024, 12, 2303322. |
| [1] | (c) Di, J. Q.; Han, S. R.; Chen, P. Chin. J. Chem. 2025, 43, 219. |
| [1] | (d) Song, S. Q.; Han, X.; Huo, Z. Z.; Yip, C. F.; Hong, X. F.; Ding, M. N.; Zheng, Y. X. Sci. China Chem. 2024, 67, 2257. |
| [2] | (a) Zhang, M.; Guo, Q.; Li, Z.; Zhou, Y.; Zhao, S.; Tong, Z.; Wang, Y.; Li, G.; Jin, S.; Zhu, M.; Zhuang, T.; Yu, S. Sci. Adv. 2023, 9, eadi9944. |
| [2] | (b) Furumi, S. Chem. Rec. 2010, 10, 394. |
| [2] | (c) Meng, G.; Zhou, J.; Han, X.; Zhao, W.; Zhang, Y.; Li, M.; Chen, C. F.; Zhang, D.; Duan, L. Adv. Mater. 2024, 36, 2307420. |
| [2] | (d) Zhang, D.; Li, M.; Chen, C. F. Chem. Soc. Rev. 2020, 49, 1331. |
| [2] | (f) Yuan, L.; Zhang, Y. P.; Zheng, Y. X. Sci. China Chem. 2024, 67, 1097. |
| [2] | (g) Zhao, C. Y.; Ji, L. K.; Ouyang, G. H.; Liu, M. H. Sci. China Chem. 2024, 54, 1380 (in Chinese). |
| [2] | ( 赵晨阳, 冀璐康, 欧阳光辉, 刘鸣华, 中国科学:化学, 2024, 54, 1380.) |
| [3] | (a) Ma, J. L.; Peng, Q.; Zhao, C. H. Chem.-Eur. J. 2019, 25, 15441. |
| [3] | (b) Li, M.; Li, S. H.; Zhang, D.; Cai, M.; Duan, L.; Fung, M. K.; Chen, C. F. Angew. Chem., Int. Ed. 2018, 57, 2889. |
| [3] | (c) Zinna, F.; Voci, S.; Arrico, L.; Brun, E.; Homberg, A.; Bouffier, L.; Funaioli, T.; Lacour, J.; Sojic, N.; Di Bari, L. Angew. Chem., Int. Ed. 2019, 58, 6952. |
| [3] | (d) Liang, Z. P.; Tang, R.; Qiu, Y. C.; Wang, Y.; Lu, H. B.; Wu, Z. G. Acta Chim. Sinica 2021, 79, 1401 (in Chinese). |
| [3] | ( 梁志鹏, 唐瑞, 邱雨晨, 王阳, 陆洪彬, 吴正光, 化学学报, 2021, 79, 1401.) |
| [3] | (e) Ren, S. Z.; Liu, Z. F.; Li, P. H.; Liu, H. D.; Lu, M. S.; Wang, K.; Yao, J. N.; Dong, H. Y.; Yang, Q. Z.; Zhang, S. D. Angew. Chem. Int. Ed. 2024, 64, e202415092. |
| [3] | (f) Zhang, L.; Wang, Y. F.; Li, M.; Gao, Q. Y.; Chen, C. F. Chin. Chem. Lett. 2021, 32, 740. |
| [3] | (g) Song, F.; Xu, Z.; Zhang, Q.; Zhao, Z.; Zhang, H.; Zhao, W.; Qiu, Z.; Qi, C.; Zhang, H.; Sung, H. H. Y.; Williams, I. D.; Lam, J. W. Y.; Zhao, Z.; Qin, A.; Ma, D.; Tang, B. Z. Adv. Funct. Mater. 2018, 28, 1800062. |
| [4] | (a) Li, B.; Li, Y.; Chan, M. H. Y.; Yam, V. W. W. J. Am. Chem. Soc. 2021, 143, 21676. |
| [4] | (b) Schnable, D.; Schley, N. D.; Ung, G. J. Am. Chem. Soc. 2022, 144, 10718. |
| [4] | (c) Ouyang, G.; Rühe, J.; Zhang, Y.; Lin, M.; Liu, M.; Würthner, F. Angew. Chem. Int. Ed. 2022, 61, e202206706. |
| [4] | (d) Sun, Y. M.; Jiang, Y. Q.; Jiang, J.; Li, T. S.; Liu, M. H. Chin. Chem. Lett. 2024, 35, 366. |
| [4] | (e) Gan, F. W.; Qiu, H. B. Chin. J. Org. Chem. 2023, 43, 371 (in Chinese). |
| [4] | ( 干富伟, 邱惠斌, 有机化学, 2023, 43, 371.) |
| [5] | (a) Richardson, F. S.; Riehl, J. P. Chem. Rev. 1977, 77, 773. |
| [5] | (b) Arrico, L.; Bari, L. D.; Zinna, F. Chem. Eur. J. 2021, 27, 2920. |
| [5] | (c) Zinna, F.; Di Bari, L. Chirality 2015, 27, 1. |
| [6] | (a) Liu, D. H.; Sun, Z. B.; Zhao, Z. H.; Peng, Q.; Zhao, C. H. Chem. Eur. J. 2019, 25, 10179. |
| [6] | (b) Wu, Z. G.; Han, H. B.; Yan, Z. P.; Luo, X. F.; Wang, Y.; Zheng, Y. X.; Zuo, J. L.; Pan, Y. Adv. Mater. 2019, 31, 1900524. |
| [6] | (c) Liu, B.; Chen, P. K. Acta Chim. Sinica 2022, 80, 929 (in Chinese). |
| [6] | ( 刘斌, 陈磅宽, 化学学报, 2022, 80, 929.) |
| [7] | (a) He, Q.; Lin, H.; Weng, Y.; Zhang, B.; Wang, Z.; Lei, G.; Wang, L.; Qiu, Y.; Bai, F. Adv. Funct. Mater. 2006, 16, 1343. |
| [7] | (b) Sánchez-Carnerero, E. M.; Moreno, F.; Maroto, B. L.; Agarrabeitia, A. R.; Ortiz, M. J.; Vo, B. G.; Muller, G.; Moya, S. D. L. J. Am. Chem. Soc. 2014, 136, 3346. |
| [8] | (a) Sun, Z. B.; Liu, J. K.; Yuan, D. F.; Zhao, Z. H.; Zhu, X. Z.; Liu, D. H.; Peng, Q.; Zhao, C. H. Angew. Chem. Int. Ed. 2019, 58, 4840. |
| [8] | (b) Xue, P. C.; Yao, B. Q.; Liu, X. H.; Sun, J. B.; Gong, P.; Zhang, Z. Q.; Qian, C.; Zhang, Y.; Lu, R. J. Mater. Chem. C 2015, 3, 1018. |
| [9] | (a) Horibe, T.; Nakagawa, K.; Hazeyama, T.; Takeda, K.; Ishihara, K. Chem. Commun. 2019, 55, 136770. |
| [9] | (b) MacLean, M. W.; Wood, T. K.; Wu, G.; Lemieux, R. P.; Crudden, C. M. Chem. Mater. 2014, 26, 5852. |
| [10] | (a) Zhang, K.; Zhao, J. Y.; Zhang, N.; Chen, J. F.; Wang, N.; Yin, X. D.; Zheng, X. Y.; Chen, P. K. J. Mater. Chem. C 2022, 10, 1816. |
| [10] | (b) Tian, G. Q.; Chen, J. F.; Zhang, K.; Shi, Y. F.; Li, C. L.; Yin, X. D.; Liu, K. L.; Chen, P. K. Inorg. Chem. 2022, 61, 15315. |
| [10] | (c) Zhang, K.; Hao, M. Y.; Jin, T. Y.; Shi, Y. F.; Tian, G. Q.; Li, C. L.; Ma, H. W.; Zhang, N.; Li, Q. S.; Chen, P. K. Chem. Eur. J. 2023, 30, e202302950. |
/
| 〈 |
|
〉 |