

Effect of Crystal Phase Ratio of Bi-Phase Titania on Structure and Catalytic Performance of Pt-WOx/TiO2 Catalyst in Glycerol Hydrogenolysis
Received date: 2025-01-13
Online published: 2025-03-25
Supported by
National Natural Science Foundation of China(22272030); Science and Technology Commission of Shanghai Municipality(2024DZSYS02)
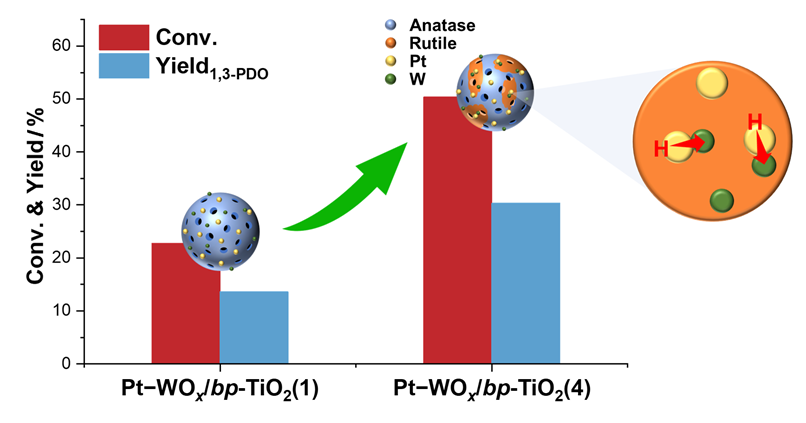
Driven by advances in the biodiesel industry, converting glycerol, the major byproduct, into valuable chemicals such as 1,3-propanediol (1,3-PDO) via selective hydrogenolysis has emerged as an important research topic. However, there is a dearth in the study of the effect of the bi-phase support on the catalytic performance of the Pt-WOx-based catalyst in glycerol hydrogenolysis. In this contribution, we synthesized bi-phase TiO2 (bp-TiO2) materials with varying rutile to anatase phase ratios using a coordination-mediated self-assembly method by adjusting the amount of HCl added. These materials were then used as supports for Pt-WOx catalysts to investigate the effect of crystal phase composition of the support on glycerol hydrogenolysis to 1,3-PDO. The Pt-WOx/bp-TiO2 catalysts were systematically characterized by techniques including X-ray diffraction (XRD), Raman spectrometry, N2 physisorption, CO pulsed adsorption, transmission electron microscopy (TEM), pyridine adsorption-Fourier transform infrared spectrometry (Py-IR), temperature-programmed desorption of NH3 (NH3-TPD), and H2 chemisorption, focusing on the phase composition, distribution and dispersion of the Pt and WOx species, acidic property, and ability of hydrogen spillover. The XRD results revealed that with the increase in the HCl dosage, the content of the rutile phase in the support increased first, and then decreased, reaching a maximum of 29%. The TEM and X-ray photoelectron spectroscopy (XPS) results disclosed that the Pt particles and WOx species were inclined to distribute on the rutile phase. The H2 chemisorption results demonstrated that the introduction of the rutile phase greatly enhanced the hydrogen spillover ability of the catalysts. In glycerol hydrogenolysis, it is identified that the glycerol conversion generally improved with the increase in the content of the rutile phase in the support, while the selectivity to 1,3-PDO remained virtually constant at around 60%. Over the Pt-WOx/bp-TiO2(4) catalyst with the highest rutile content of 29%, the yield of 1,3-PDO reached the highest value of 30.3%. This catalyst also displayed good stability. It is plausible that the preferential distribution of the Pt and WOx species on the rutile surface is conducive to the formation of more Pt-WOx interfaces, which greatly enhances hydrogen spillover and thus boosts the yield of 1,3-PDO. This work elucidates the important role of the phase composition of bi-phase TiO2 support on the catalytic performance of the Pt-WOx-based catalyst in glycerol selective hydrogenolysis, which opens up new avenue for the design of high-performance glycerol hydrogenolysis catalysts by means of engineering the phase composition of the support.
Jianhua Chen , Lan Jiang , Yang Zeng , Songhai Xie , Yan Pei , Minghua Qiao . Effect of Crystal Phase Ratio of Bi-Phase Titania on Structure and Catalytic Performance of Pt-WOx/TiO2 Catalyst in Glycerol Hydrogenolysis[J]. Acta Chimica Sinica, 2025 , 83(4) : 332 -340 . DOI: 10.6023/A25010018
| [1] | Hoekman, S. K.; Broch, A.; Robbins, C.; Ceniceros, E.; Natarajan, M. Renewable Sustainable Energy Rev. 2012, 16, 143. |
| [2] | Attarbachi, T.; Kingsley, M. D.; Spallina, V. Fuel 2023, 340, 127485. |
| [3] | Kaur, G.; Srivastava, A. K.; Chand, S. Biochem. Eng. J. 2012, 64, 106. |
| [4] | Zhu, F.; Liu, D.; Chen, Z. Green Chem. 2022, 24, 1390. |
| [5] | Shrirame, B. S.; Varma, A. R.; Sahoo, S. S.; Gayen, K.; Maity, S. K. Biomass Bioenergy 2023, 177, 106943. |
| [6] | Bhowmik, S.; Darbha, S. Catal. Rev. 2021, 63, 639. |
| [7] | Yang, M.; Jiao, Y.; Ren, Y. Prog. Chem. 2024, 36, 256. |
| [8] | Wu, F.; Jiang, H.; Zhu, X.; Lu, R.; Shi, L.; Lu, F. ChemSusChem 2021, 14, 569. |
| [9] | Fan, Y.; Cheng, S.; Wang, H.; Tian, J.; Xie, S.; Pei, Y.; Qiao, M.; Zong, B. Appl. Catal., B 2017, 217, 331. |
| [10] | Zeng, Y.; Jiang, L.; Zhang, X.; Xie, S.; Pei, Y.; Zhou, G.; Hua, W.; Qiao, M.; Li, Z.; Zong, B. ACS Sustainable Chem. Eng. 2022, 10, 9532. |
| [11] | Wang, Y.; Zhou, Z.; Wang, C.; Zhao, L.; Xia, Q. Front. Chem. 2022, 10, 1004925. |
| [12] | Zhou, G.; Dou, R.; Bi, H.; Xie, S.; Pei, Y.; Fan, K.; Qiao, M.; Sun, B.; Zong, B. J. Catal. 2015, 332, 119. |
| [13] | Feng, Y.; Zhang, Y.; Wang, J.; Ling, L.; Zhang, R.; Fan, M.; Hou, B.; Li, D.; Wang, B. ACS Catal. 2024, 14, 1874. |
| [14] | Zhang, W.; He, H.; Tian, Y.; Lan, K.; Liu, Q.; Wang, C.; Liu, Y.; Elzatahry, A.; Che, R.; Li, W.; Zhao, D. Chem. Sci. 2019, 10, 1664. |
| [15] | Zhang, H.; Banfield, J. F. J. Phys. Chem. B 2000, 104, 3481. |
| [16] | Ohsaka, T.; Izumi, F.; Fujiki, Y. J. Raman Spectrosc. 1978, 7, 321. |
| [17] | Deo, G.; Turek, A. M.; Wachs, I. E.; Machej, T.; Haber, J.; Das, N.; Eckert, H.; Hirt, A. M. Appl. Catal. A 1992, 91, 27. |
| [18] | Yan, J.; Wu, G.; Guan, N.; Li, L.; Li, Z.; Cao, X. Phys. Chem. Chem. Phys. 2013, 15, 10978. |
| [19] | Kim, D. S.; Ostromecki, M.; Wachs, I. E. J. Mol. Catal. A 1996, 106, 93. |
| [20] | Arribas, M. A.; Márquez, F.; Martínez, A. J. Catal. 2000, 190, 309. |
| [21] | Komornicki, S.; Radecka, M.; Sobas, P. J. Mater. Sci.: Mater. Electron. 2004, 15, 527. |
| [22] | Kim, T. Y.; Park, D. S.; Choi, Y.; Baek, J.; Park, J. R.; Yi, J. J. Mater. Chem. 2012, 22, 10021. |
| [23] | Ou, G.; Xu, Y.; Wen, B.; Lin, R.; Ge, B.; Tang, Y.; Liang, Y.; Yang, C.; Huang, K.; Zu, D.; Yu, R.; Chen, W.; Li, J.; Wu, H.; Liu, L.-M.; Li, Y. Nat. Commun. 2018, 9, 1302. |
| [24] | He, J.; Burt, S. P.; Ball, M.; Zhao, D.; Hermans, I.; Dumesic, J. A.; Huber, G. W. ACS Catal. 2018, 8, 1427. |
| [25] | Zhu, S.; Qiu, Y.; Zhu, Y.; Hao, S.; Zheng, H.; Li, Y. Catal. Today 2013, 212, 120. |
| [26] | García-Fernández, S.; Gandarias, I.; Requies, J.; Güemez, M. B.; Bennici, S.; Auroux, A.; Arias, P. L. J. Catal. 2015, 323, 65. |
| [27] | Lauriol-Garbey, P.; Postole, G.; Loridant, S.; Auroux, A.; Belliere-Baca, V.; Rey, P.; Millet, J. M. M. Appl. Catal. B 2011, 106, 94. |
| [28] | Jiang, F.; Zeng, L.; Li, S.; Liu, G.; Wang, S.; Gong, J. ACS Catal. 2014, 5, 438. |
| [29] | Gong, L.; Lu, Y.; Ding, Y.; Lin, R.; Li, J.; Dong, W.; Wang, T.; Chen, W. Appl. Catal. A 2010, 390, 119. |
| [30] | Deng, C.; Duan, X.; Zhou, J.; Chen, D.; Zhou, X.; Yuan, W. Catal. Today 2014, 234, 208. |
| [31] | Zhao, B.; Liang, Y.; Liu, L.; He, Q.; Dong, J. Green Chem. 2020, 22, 8254. |
| [32] | Wen, Y.; Shen, W.; Li, Y.; Fang, Y. React. Kinet., Mech. Catal. 2021, 132, 219. |
| [33] | Chen, J.; Huang, H.; Pan, H.; Cao, Y.; Jiang, L.; Xia, Q.; Meng, X.; Qiu, J.; Zuilhof, H.; Liu, S. ACS Sustainable Chem. Eng. 2024, 12, 6242. |
| [34] | Cheng, S.; Zeng, Y.; Pei, Y.; Fan, K.; Qiao, M.; Zong, B. Acta Chim. Sinica 2019, 77, 1054. (in Chinese) |
| [34] | (成诗婕, 曾杨, 裴燕, 范康年, 乔明华, 宗保宁, 化学学报, 2019, 77, 1054.) |
| [35] | Zeng, Y.; Jiang, L.; Zhang, X.; Xie, S.; Pei, Y.; Qiao, M.; Li, Z.; Xu, H.; Fan, K.; Zong, B. Acta Chim. Sinica 2022, 80, 903. (in Chinese) |
| [35] | (曾杨, 姜兰, 张晓昕, 谢颂海, 裴燕, 乔明华, 李振华, 徐华龙, 范康年, 宗保宁, 化学学报, 2022, 80, 903.) |
| [36] | Jiang, L.; Fan, Y.; Zhang, X.; Pei, Y.; Yan, S.; Qiao, M.; Fan, K.; Zong, B. Acta Chim. Sinica 2023, 81, 231. (in Chinese) |
| [36] | (姜兰, 范义秋, 张晓昕, 裴燕, 闫世润, 乔明华, 范康年, 宗保宁, 化学学报, 2023, 81, 231.) |
| [37] | Wang, L.; Stuckert, A. N.; Chen, H.; Yang, R. T. J. Phys. Chem. C 2011, 115, 4793. |
| [38] | Pevzner, S.; Pri-Bar, I.; Lutzky, I.; Ben-Yehuda, E.; Ruse, E.; Regev, O. J. Phys. Chem. C 2014, 118, 27164. |
| [39] | Fan, Y.; Cheng, S.; Wang, H.; Ye, D.; Xie, S.; Pei, Y.; Hu, H.; Hua, W.; Li, Z. H.; Qiao, M.; Zong, B. Green Chem. 2017, 19, 2174. |
| [40] | Lei, N.; Zhao, X.; Hou, B.; Yang, M.; Zhou, M.; Liu, F.; Wang, A.; Zhang, T. ChemCatChem 2019, 11, 3903. |
| [41] | Zhou, W.; Zhao, Y.; Wang, Y.; Wang, S.; Ma, X. ChemCatChem 2016, 8, 3663. |
| [42] | Conner, W. C., Jr.; Falconer, J. L. Chem. Rev. 1995, 95, 759. |
/
| 〈 |
|
〉 |