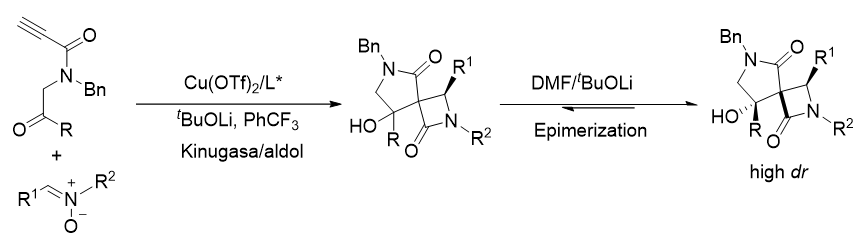
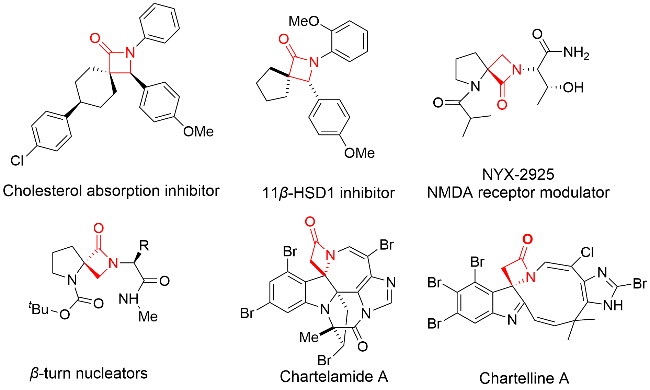
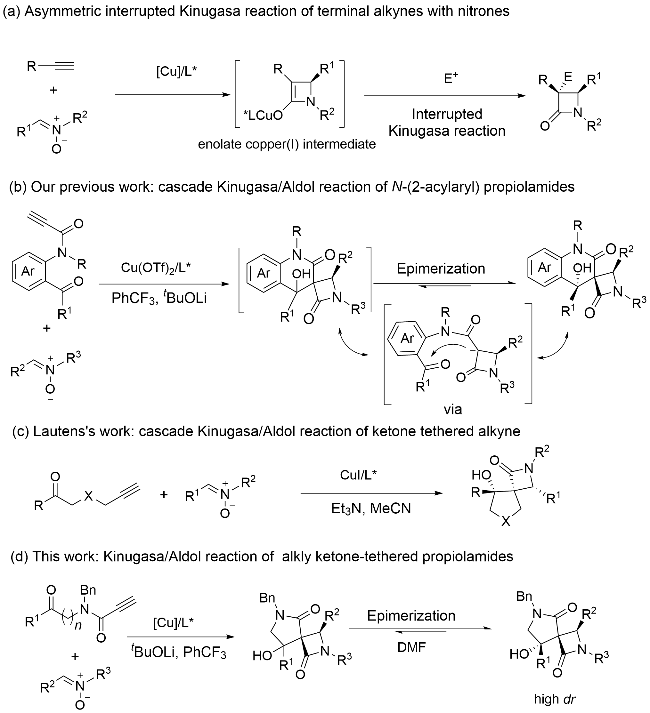
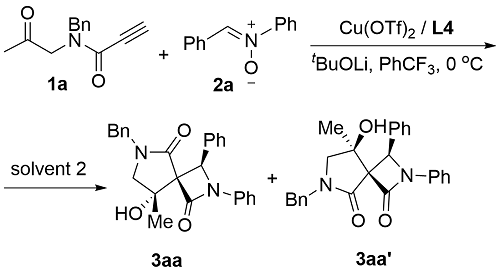
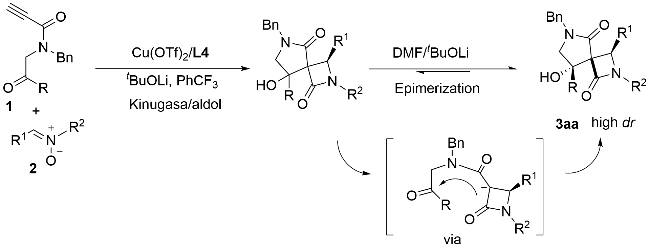
1 引言
2 结果与讨论
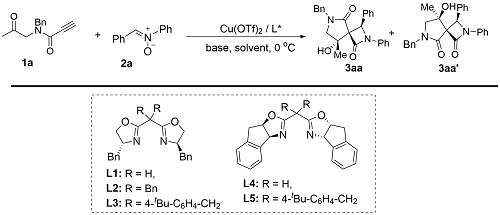
2.1 反应条件筛选
表1 反应条件优化aTable 1 Optimization of reaction conditionsa |
| Entry | L* | Solvent | Base | Yieldb/% | drc (3aa/3aa') | erd (3aa) |
|---|---|---|---|---|---|---|
| 1 | L1 | PhCF3 | tBuOLi | 41 | 4∶1 | 62∶38 |
| 2 | L2 | PhCF3 | tBuOLi | 39 | 10∶1 | 53∶47 |
| 3 | L3 | PhCF3 | tBuOLi | 58 | 8∶1 | 55∶45 |
| 4 | L4 | PhCF3 | tBuOLi | 66 | 3∶1 | 90∶10 |
| 5 | L5 | PhCF3 | tBuOLi | 58 | 7∶1 | 55∶45 |
| 6 | L4 | MeCN | tBuOLi | 51 | 1∶1 | 53∶47 |
| 7 | L4 | DCE | tBuOLi | 36 | 6∶1 | 64∶36 |
| 8 | L4 | 1,4-dioxane | tBuOLi | 47 | 2∶1 | 86∶14 |
| 9 | L4 | THF | tBuOLi | 41 | 3∶1 | 88∶12 |
| 10 | L4 | DMF | tBuOLi | n.d. | — | — |
| 11 | L4 | PhCF3 | K2CO3 | 46 | 3∶1 | 80∶20 |
| 12 | L4 | PhCF3 | Cs2CO3 | 44 | 2∶1 | 85∶15 |
| 13 | L4 | PhCF3 | NaH | 35 | 3∶1 | 75∶25 |
a Reaction conditions: 1a (0.2 mmol), 2a (0.24 mmol), Cu(OTf)2 (10 mol%), ligand (12 mol%), base (0.3 mmol), solvent (2 mL), 0 ℃, 12 h. b Isolated yields of 3aa/3aa'. c Determined by 1H NMR analysis of the crude reaction mixture. d Determined by HPLC analysis. |
表2 反应条件进一步优化aTable 2 Further optimization of reaction conditionsa |
| Entry | Solvent 2 | Yieldb/% | drc (3aa/3aa') |
|---|---|---|---|
| 1 | PhCF3 | 46 | 3∶1 |
| 2 | DCE | 36 | 4∶1 |
| 3 | THF | 38 | 3∶1 |
| 4 | MeCN | 41 | 6∶1 |
| 5 | 1,4-dioxane | 46 | 3∶1 |
| 6 | DMF | 51 | 9∶1 |
a Reaction conditions: 1a (0.2 mmol), 2a (0.24 mmol), Cu(OTf)2 (10 mol%), L4 (12 mol%), tBuOLi (0.3 mmol), PhCF3 (2 mL), 0 ℃, 12 h. then evaporated the PhCF3 and added solvent 2, for 5 h at room temperature. b Isolated yields of 3aa/3aa'. c Determined by 1H NMR analysis of the crude reaction mixture. |
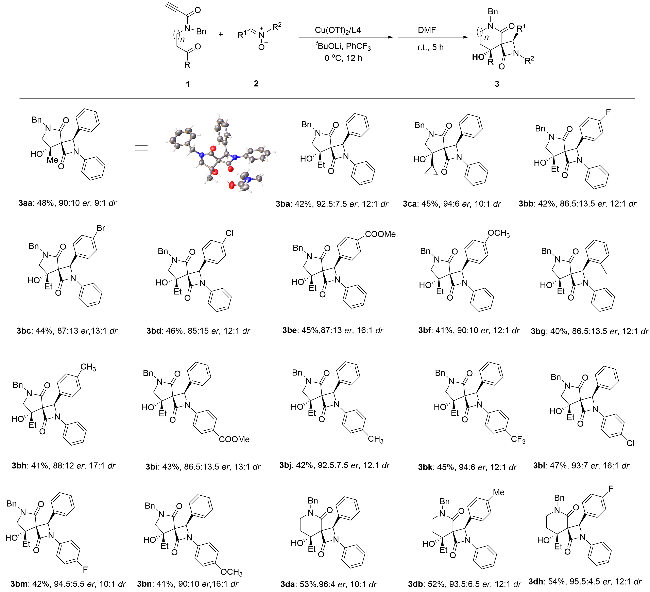
2.2 底物范围
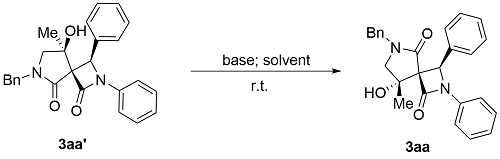
2.3 控制实验
表3 控制实验Table 3 Control experiment |
| Entry | Base | Solvent | Conversion rateb |
|---|---|---|---|
| 1 | tBuOLi | DMF | ≈85% |
| 2 | Et3N | DMF | n.d. |
| 3 | Cs2CO3 | DMF | ≈15% |
| 4 | tBuOLi | PhCF3 | n.d. |