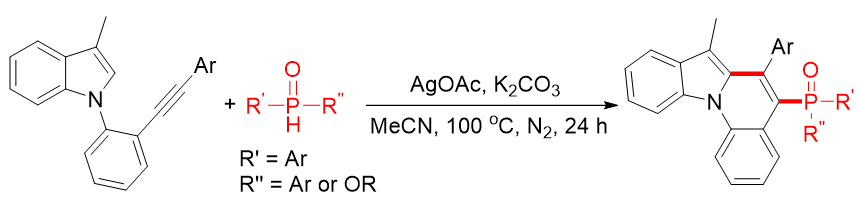
Nitrogen-containing heterocyclic compounds, especially indolo[1,2-a]quinolines with unique tetracyclic frameworks, are widely present in natural products, bioactive substances, and functional materials, exhibiting significant potential in pharmaceuticals and materials science. Meanwhile, heterocyclic organophosphorus compounds have attracted considerable attention due to their extensive applications in organic synthesis, medicinal chemistry, and materials science, and modifying organophosphorus functionalities can enhance biological activities. Herein, a highly efficient and innovative synthetic methodology for the preparation of non-coplanar diarylphosphinoyl indolo[1,2-a]quinolines from indoloalkynes and secondary diarylphosphine oxides has been successfully developed.
This approach entails a silver-mediated cascade process, involving the generation of diarylphosphinoyl radicals with significant steric hindrance via the reaction of silver salts (AgOAc as the optimal oxidant) with secondary diarylphosphine oxides, followed by the addition of these radicals to indoloalkynes to form alkenyl radical intermediates, and subsequent intramolecular radical cyclization, oxidation, and deprotonation to construct C-P and C-C bonds. The optimized reaction conditions are determined as: indoloalkynes (1.0 equiv), secondary diarylphosphine oxides (2.0 equiv), AgOAc (3.0 equiv), K3PO4 (2.5 equiv) as the base, MeCN as the solvent, reaction at 100 °C under a nitrogen atmosphere for 24 hours.
Notably, this protocol exhibits excellent substrate compatibility: electron-rich, electron-deficient, and ortho-substituted aryl groups on indoloalkynes are well-tolerated, yielding corresponding products in 55-81% yields; secondary diarylphosphine oxides with electron-withdrawing groups (-F), electron-donating groups (-Me, -OMe), and even alkyl substituents can also participate in the reaction, giving products with moderate to good yields (51-79%). A gram-scale experiment under optimized conditions still affords the desired product with a 68% yield, demonstrating its potential for large-scale synthesis.
Control experiments with the free radical inhibitor TEMPO confirm that the reaction proceeds via a free radical mechanism. Single-crystal X-ray diffraction analysis of the product reveals that the four aromatic rings are non-coplanar (with a 14.899° angle between the normals of two internal aromatic rings) due to steric hindrance from the diarylphosphinoyl group, while maintaining intact aromaticity and stability.
Overall, this method features mild reaction conditions, simplicity in operation, broad substrate scope, and the ability to yield diverse non-coplanar diarylphosphinoyl indolo[1,2-a]quinolines, holding considerable potential for applications in pharmaceuticals and materials science, and representing a substantial advancement in the synthesis of such compounds.
Helin Wang
,
Qingle Zeng
. Cascade Addition and Cyclization of Indoloalkynes with Phosphine Oxides for the Synthesis of Diarylphosphinoyl Indolo[1,2-a]quinolines[J]. Acta Chimica Sinica, 0
: 4
.
DOI: 10.6023/A25080283
[1] Segraves N. L.; Robinson S. J.; Garcia D.; Said S. A.; Fu X.; Schmitz F. J.; Pietraszkiewicz H.; Valeriote F. A.; Crews, P. J. Nat. Prod.2004, 67, 783.
[2] Ishikura M.; Yamada K. J.; Abe, T. Nat. Prod. Rep.2010, 27, 1630.
[3] Sun B.; Shi R. C.; Zhang K. S.; Tang X. L.; Shi X. Y.; Xu J. Y.; Yang J.; Jin C. Chem. Commun.2021, 57, 6050.
[4] Michael, J. P. Nat. Prod. Rep.2005, 22, 603.
[5] Silva T. S.; Rodrigues M. T.; Santos H.; Zeoly L. A.; Almeida W. P.; Barcelos R. C.; Gomes R. C.; Fernandes F. S.; Coelho F.Tetrahedron 2019, 75, 2063.
[6] Brown D. W.; Graupner P. R.; Sainsbury M.; Shertzer H. G. Tetrahedron1991, 47, 4383.
[7] Lavrado J.; Moreira R.; Paulo, A. Curr. Med. Chem.2010, 17, 2348.
[8] Anderson W. K.; Heider A. R.; Raju N.; Yucht J. A.J. Med. Chem. 1988, 31, 2097.
[9] Reddy M. V.; Rao M. R.; Rhodes D.; Hansen M. S.; Rubins K.; Bushman F. D.; Venkateswarlu Y.; Faulkner D. J.J. Med. Chem. 1999, 42, 1901.
[10] Thanetchaiyakup A.; Borwornpinyo S.; Rattanarat H.; Kanjanasirirat P.; Jearawuttanakul K.; Seemakhan S.; Chuanopparat N.; Ngernmeesri P. Tetrahedron Lett.2021, 82, 153365.
[11] Xu H.; Fan L. L.Eur. J. Med. Chem. 2011, 46, 1919.
[12] Sun K.; Chen X. L.; Zhang Y. L.; Li K.; Huang X. Q.; Peng Y. Y.; Qu L. B.; Yu B. Chem. Commun.2019, 55, 12615.
[13] Wang Z.; He, W. M. Chin. J. Org. Chem.2019, 39, 3594 (in Chinese). (王峥, 何卫民. 有机化学2019, 39, 3594).
[14] Phetcharawetch J.; Uppalabat T.; Sawektreeratana N.; Suwannapaporn P.; Todsaporn D.; Rungrotmongkol T.; Muanprasat C.; Kuhakarn C. RSC Adv.2025, 15, 3139.
[15] Thanetchaiyakup, a.; Borwornpinyo, S.; Rattanarat, H.; Kanjanasirirat, P.; Jearawuttanakul, K.; Seemakhan, S.; Chuanopparat, N.; Ngernmeesri, P.Tetrahedron Lett. 2021, 82, 153365.
[16] Verma A. K.; Shukla S. P.; Singh J.; Rustagi, V. J. Org. Chem.2011, 76, 5670.
[17] Uppalabat T.; Hassa N.; Sawektreeratana N.; Leowanawat P.; Janthakit P.; Nalaoh P.; Promarak V.; Soorukram D.; Reutrakul V.; Kuhakarn, C. J. Org. Chem.2023, 88, 5403.
[18] Zhang S.; Yuan J. W.; Huang G. C.; Ma C. J.; Yang L. G.; Xiao Y. M.; Qu, L. B. J. Org. Chem.2023, 88, 11712.
[19] Gao Y.;T. G. Zhao,Y. Chin. J. Org. Chem.2018, 38, 62 (in Chinese). (高玉珍, 唐果, 赵玉芬. 有机化学2018, 38, 62).
[20] Tang W. J.; Zhang, X. M. Chem. Rev.2003, 103, 3029.
[21] Tomashenko O. A.; Grushin, V. V. Chem. Rev.2011, 111, 4475.
[22] George A.; Veis A. Chem. Rev.2008, 108, 4670.
[23] Queffélec C.; Petit M.; Janvier P.; Knight D. A.; Bujoli B. Chem. Rev.2012, 112, 3777.
[24] Bansal R. K.(Ed) Topics in Heterocyclic Chemistry, Vol 21, Springer, Berlin, 2010.
[25] Regina G. L.; Coluccia A.; Piscitelli F.; Bergamini A.; Sinistro A.; Cavazza A.; Maga G.; Samuele A.; Zanoli S.; Novellino E.; Artico M.; Silvestri, R. J. Med. Chem.2007, 50, 5034.
[26] Wang H.; Li S.; Wang B.; Li, B. Org. Chem. Front.2018, 5, 88.
[27] Xiao Q.; Tong Q.-X.; Zhong J.-J.Chin. J. Org. Chem. 2022, 42, 3979 (in Chinese). (肖潜, 佟庆笑, 钟建基. 有机化学2022, 42, 3979).
[28] Ren Z.; Luo W. Zhou,J.Chin. J. Org. Chem. 2023, 43, 2026 (in Chinese). (任志军, 罗维纬, 周俊. 有机化学2023, 43, 2026).
[29] Zeng Q.; Mi A.; Jiang Y. Prog. Chem.2004, 16, 603 (in Chinese). (曾庆乐,宓爱巧,蒋耀忠. 化学进展 2004, 16, 603).
[30] Feng J.; Zhang Q.; Li F.; Yang L.; Kuchukulla R. R.; Zeng Q. Synlett2021, 32, 224.
[31] Zeng Q.-L.; Tang H.-Y.; Zhang S.; Liu J.-C.Chin. J. Chem. 2008, 26, 1435.
[32] Zhou S.; Zeng, Q. Org. Chem. Front.2025, https://doi.org/10.1039/D5QO00681C.
[33] Jiang W.; Huang Y.; Zhou L.; Zeng, Q. Sci. China Chem.2019, 62, 1213.
[34] Yang L.; Feng J.; Qiao M.; Zeng, Q. Org. Chem. Front.2018, 5, 24.
[35] Chen S.; Zhang P.; Shu W.; Gao Y.; Tang G.; Zhao Y. Org. Lett.2016, 18, 5712.
[36] Zhang B.; Daniliuc C. G.; Studer A. Org. Lett.2014, 16, 250.
[37] Chen Y-R.; Duan, W-L. J. Am. Chem. Soc.2013, 135, 16754.


