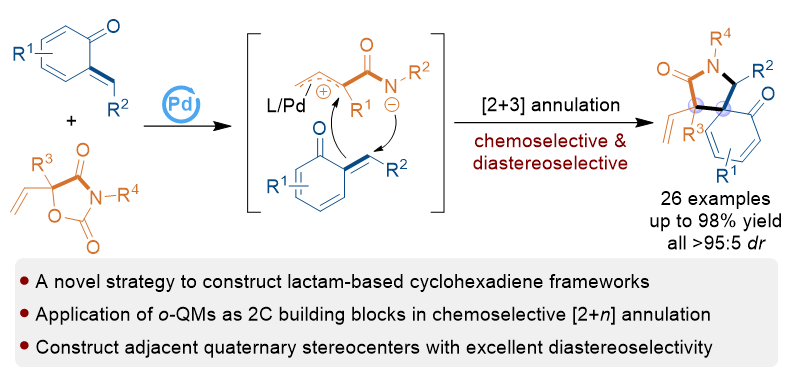
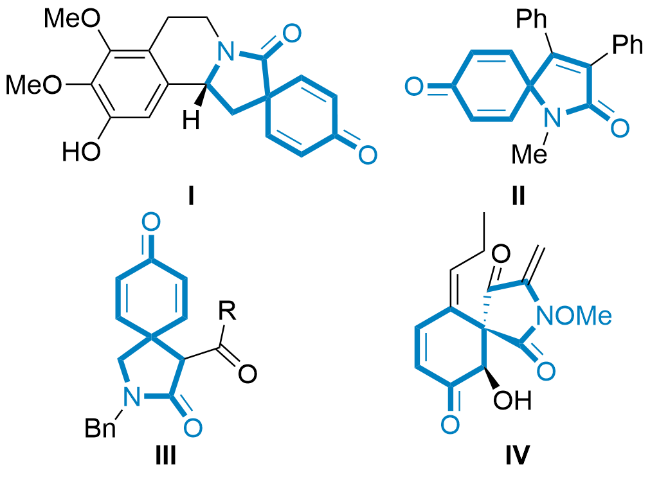
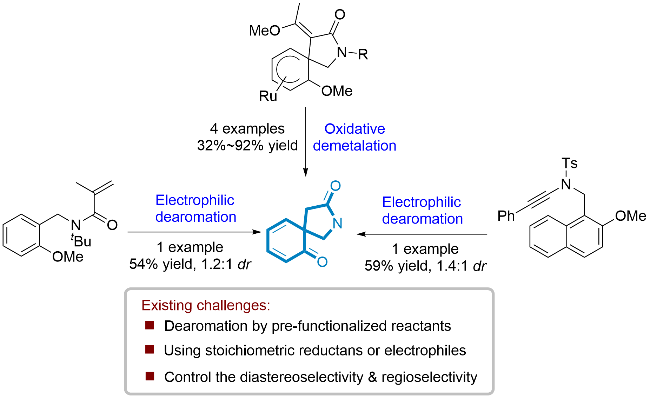
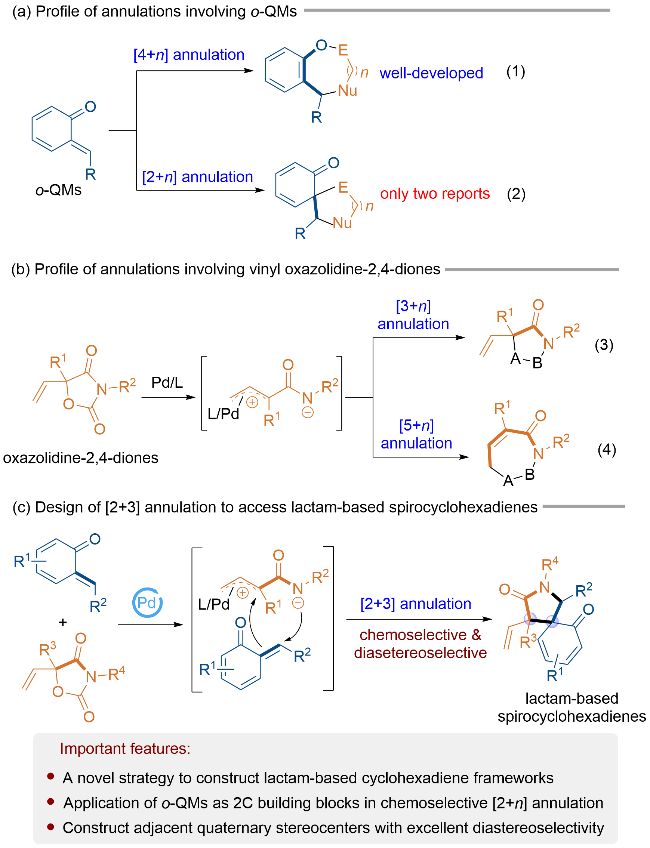
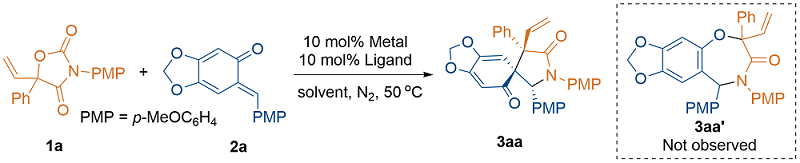
1 引言
2 结果与讨论
2.1 反应条件优化
表1 反应条件的优化aTable 1 Optimization of the reaction conditionsa |
| Entry | Metal | Ligand | Solvent | Yield/% | dr |
|---|---|---|---|---|---|
| 1 | Pd2(dba)3 | PPh3 | MeCN | 84 | >95∶5 |
| 2 | Pd(PPh3)2Cl2 | PPh3 | MeCN | 12 | >95∶5 |
| 3 | [Ir(cod)Cl]2 | PPh3 | MeCN | N.R. | — |
| 4 | CuOTf | PPh3 | MeCN | N.R. | — |
| 5 | Pd2(dba)3 | dppp | MeCN | 40 | >95∶5 |
| 6 | Pd2(dba)3 | dppf | MeCN | 36 | >95∶5 |
| 7 | Pd2(dba)3 | PPh3 | DCE | 95 | >95∶5 |
| 8 | Pd2(dba)3 | PPh3 | EtOAc | 83 | >95∶5 |
| 9 | Pd2(dba)3 | PPh3 | DMF | 62 | >95∶5 |
| 10 | Pd2(dba)3 | PPh3 | Toluene | 82 | >95∶5 |
| 11 | Pd2(dba)3 | PPh3 | THF | 76 | >95∶5 |
| 12b | Pd2(dba)3 | PPh3 | DCE | 86 | >95∶5 |
| 13c | Pd2(dba)3 | PPh3 | DCE | 68 | >95∶5 |
a Unless otherwise indicated, the reactions were performed under the conditions: 1a (0.05 mmol), 2a (0.06 mmol), metal (0.005 mmol), ligand (0.005 mmol), MeCN (0.5 mL), 50 ℃ for 16 h, isolated yields were reported. b The reaction was carried out at 30 ℃. c The reaction was carried out at 70 ℃. N.R.=No reaction. |
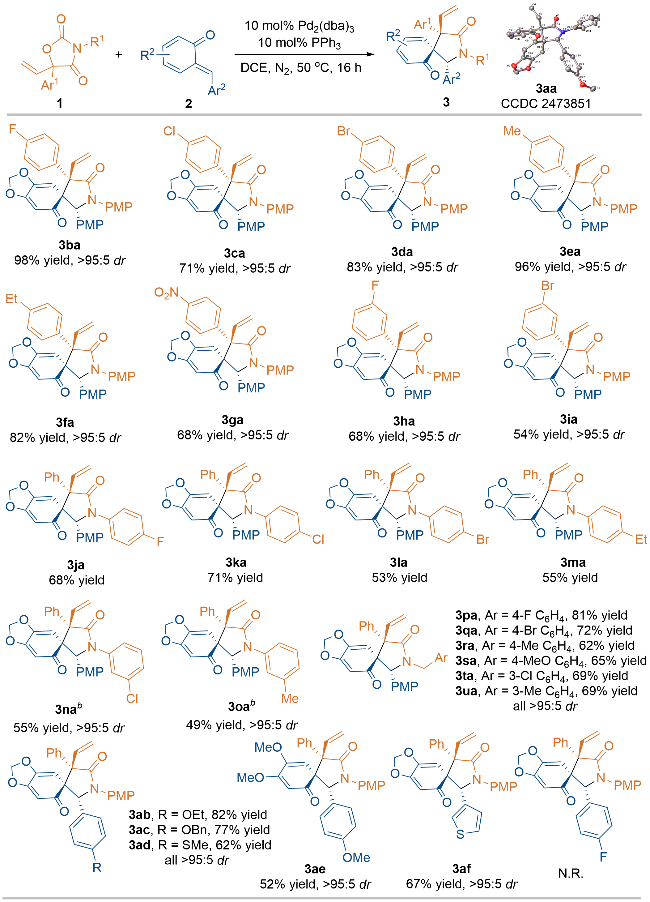
2.2 反应底物普适性考察
图式3 [2+3]环化反应的底物范围aScheme 3 Substrate scope of the [2+3] annulationa a Unless otherwise indicated, the reactions were performed under the conditions: 1 (0.1 mmol), 2 (0.12 mmol), Pd2(dba)3 (0.01 mmol), PPh3 (0.01 mmol), DCE (1 mL), 50 ℃ for 16 h, isolated yields were reported. b The reaction was carried out for 24 h. |
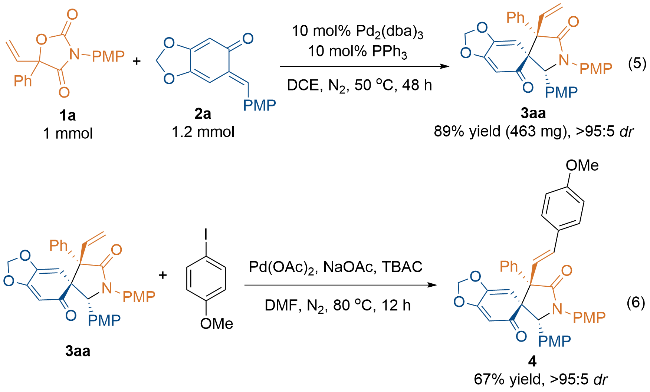
2.3 放大量与衍生化实验
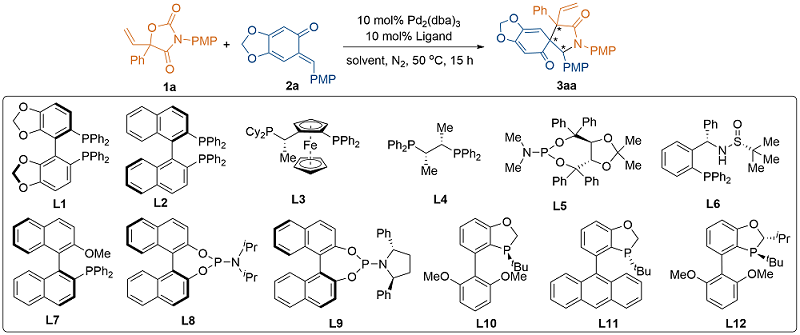
2.4 不对称催化的初步尝试
表2 催化不对称[2+3]环化反应的初步探索aTable 2 Preliminary investigation on the catalytic asymmetric [2+3] annulationa |
| Entry | Ligand | Solvent | Yield/% | dr | ee/% |
|---|---|---|---|---|---|
| 1 | L1 | MeCN | N.R. | — | — |
| 2 | L2 | MeCN | N.R. | — | — |
| 3 | L3 | MeCN | N.R. | — | — |
| 4 | L4 | MeCN | N.R. | — | — |
| 5 | L5 | MeCN | 80 | >95∶5 | 29 |
| 6 | L6 | MeCN | 23 | >95∶5 | -13 |
| 7 | L7 | MeCN | 67 | >95∶5 | 15 |
| 8 | L8 | MeCN | 88 | >95∶5 | -15 |
| 9 | L9 | MeCN | 62 | >95∶5 | -7 |
| 10 | L10 | MeCN | 72 | >95∶5 | 19 |
| 11 | L11 | MeCN | 41 | >95∶5 | 13 |
| 12 | L12 | MeCN | 25 | >95∶5 | 42 |
| 13 | L12 | DCE | 35 | >95∶5 | 9 |
| 14 | L12 | DMF | 14 | >95∶5 | 12 |
| 15 | L12 | THF | 49 | >95∶5 | 13 |
| 16 | L12 | Toluene | 58 | >95∶5 | 14 |
a Unless otherwise indicated, the reactions were performed under the conditions: 1a (0.05 mmol), 2a (0.06 mmol), Pd2(dba)3 (0.005 mmol), ligand (0.005 mmol), MeCN (0.5 mL), 50 ℃ for 15 h, isolated yields were reported. The ee value was determined by HPLC. The dr value was determined by NMR. |