Chinese Journal of Organic Chemistry ›› 2021, Vol. 41 ›› Issue (4): 1658-1669.DOI: 10.6023/cjoc202009048 Previous Articles Next Articles
Original article
收稿日期:2020-09-22
修回日期:2020-10-23
发布日期:2020-12-10
通讯作者:
雷川虎
基金资助:
Danfeng Yea,b, Hao Chenb, Zhiyuan Liub, Chuanhu Leib,*( )
)
Received:2020-09-22
Revised:2020-10-23
Published:2020-12-10
Contact:
Chuanhu Lei
About author:Supported by:Share
Danfeng Ye, Hao Chen, Zhiyuan Liu, Chuanhu Lei. Transamidation of N-Benzyl-N-Boc-amides under Transition Metal-Free and Base-Free Conditions[J]. Chinese Journal of Organic Chemistry, 2021, 41(4): 1658-1669.
| Entry | Cs2CO3/ equiv. | Solvent | T/℃ | Conv./% of 1aa | Yielda/% of 3a |
|---|---|---|---|---|---|
| 1 | 2.0 | DMF | 100 | 41 | 33 |
| 2 | 1.0 | DMF | 100 | 70 | 48 |
| 3 | 0.2 | DMF | 100 | 85 | 63 |
| 4 | 0 | DMF | 100 | 84 | 81 |
| 5 | 0 | THF | Reflux | 97 | 83 |
| 6 | 0 | CH3CN | Reflux | 79 | 78 |
| 7 | 0 | Ether | Reflux | 93 | 81 |
| 8 | 0 | DCM | Reflux | 7 | 6 |
| 9 | 0 | Toluene | 100 | 91 | 91 |
| 10 | 0 | Toluene | 80 | 54 | 50 |
| 11 | 0 | Toluene | Reflux | 99 | 97 (90)b |
| 12c | 0 | Toluene | Reflux | 86 | 79 |
| Entry | Cs2CO3/ equiv. | Solvent | T/℃ | Conv./% of 1aa | Yielda/% of 3a |
|---|---|---|---|---|---|
| 1 | 2.0 | DMF | 100 | 41 | 33 |
| 2 | 1.0 | DMF | 100 | 70 | 48 |
| 3 | 0.2 | DMF | 100 | 85 | 63 |
| 4 | 0 | DMF | 100 | 84 | 81 |
| 5 | 0 | THF | Reflux | 97 | 83 |
| 6 | 0 | CH3CN | Reflux | 79 | 78 |
| 7 | 0 | Ether | Reflux | 93 | 81 |
| 8 | 0 | DCM | Reflux | 7 | 6 |
| 9 | 0 | Toluene | 100 | 91 | 91 |
| 10 | 0 | Toluene | 80 | 54 | 50 |
| 11 | 0 | Toluene | Reflux | 99 | 97 (90)b |
| 12c | 0 | Toluene | Reflux | 86 | 79 |
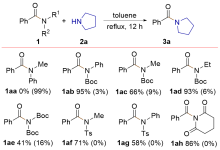




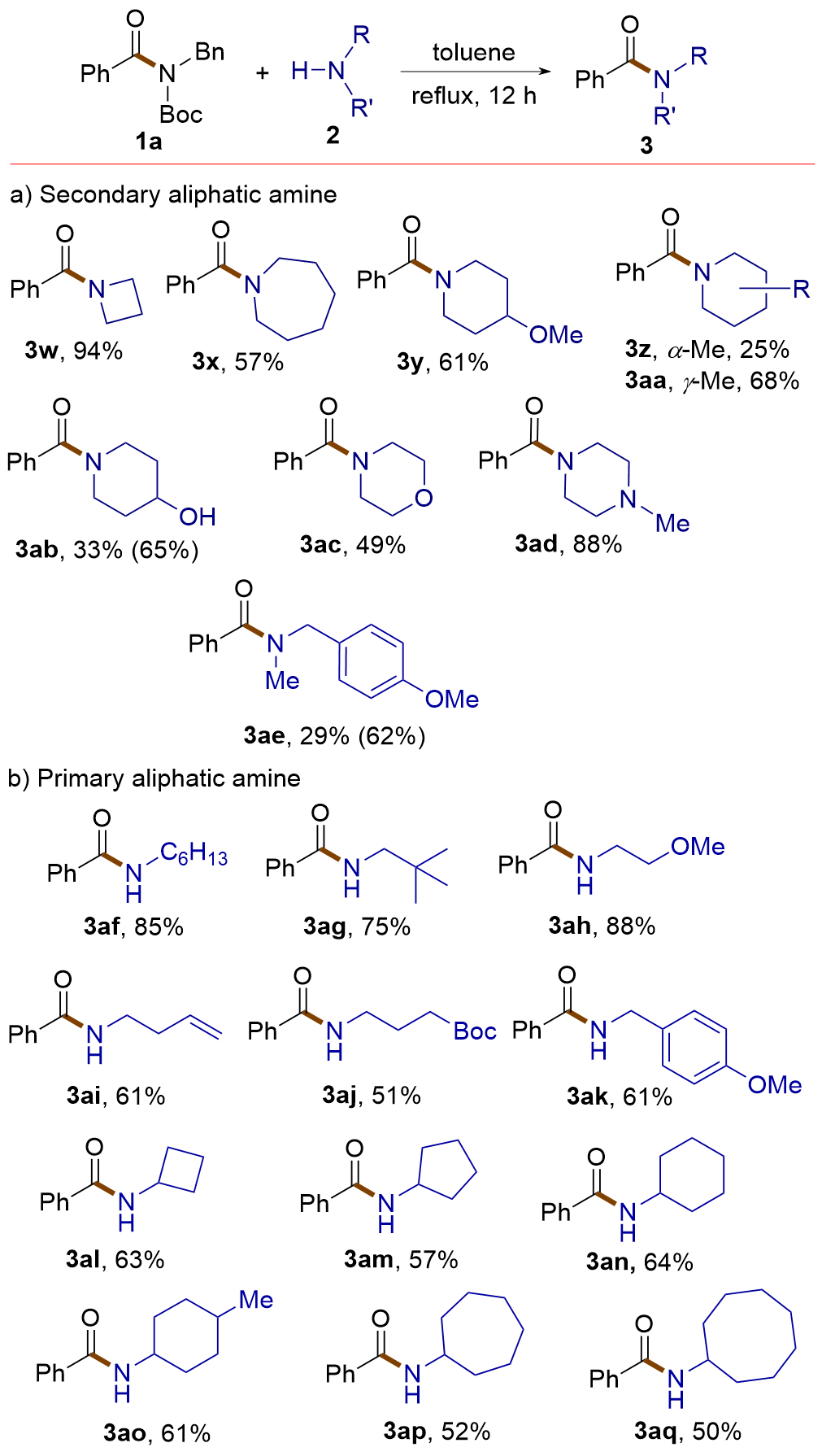
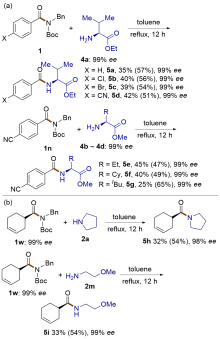
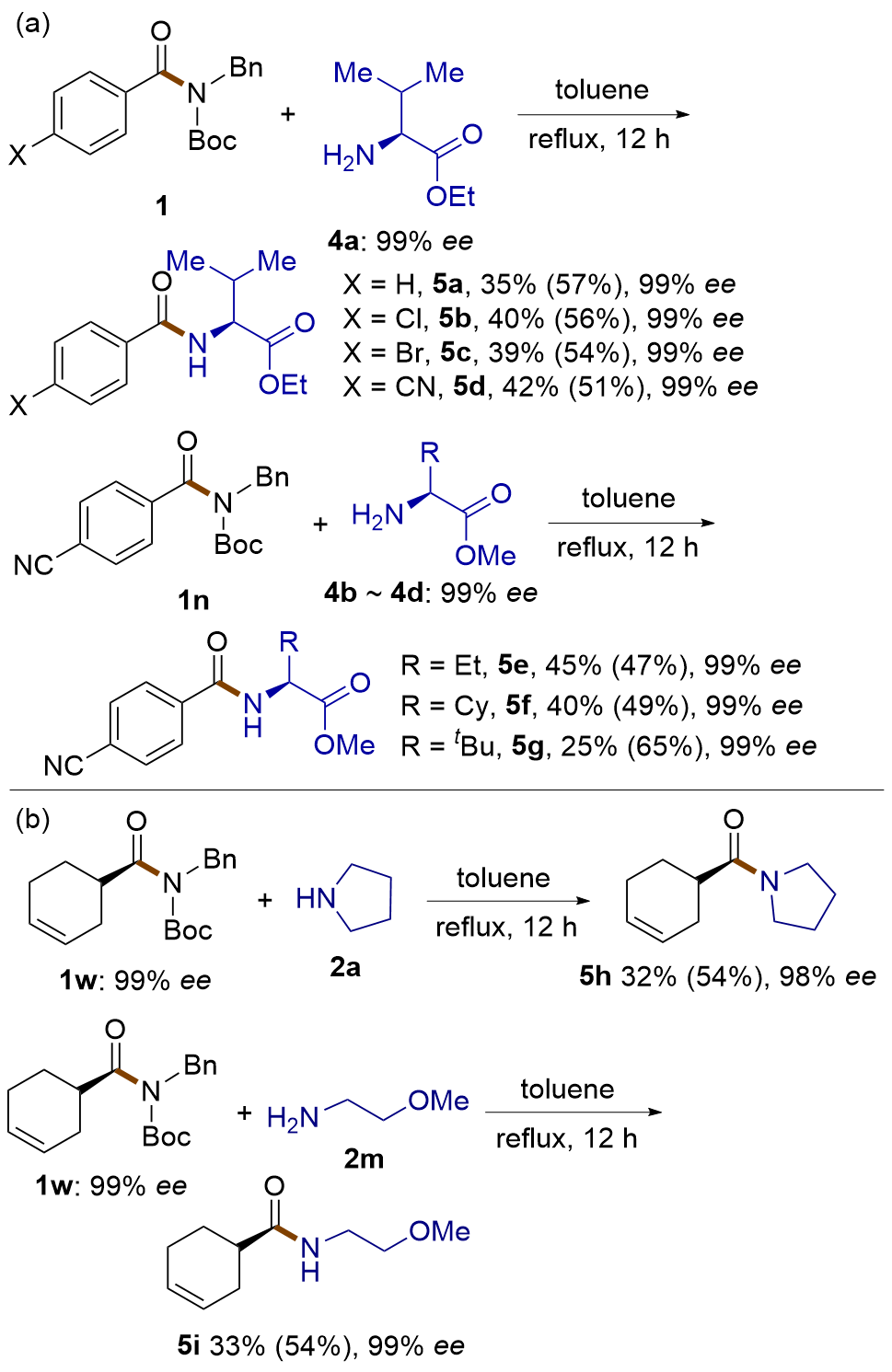
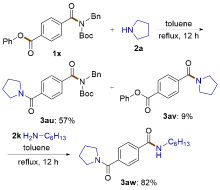



| [1] |
(a) Greenberg, A.R.; Breneman, C.M.; Liebman, J.F. The Amide Linkage: Structural Significance in Chemistry, Biochemistry, and Materials Science, Wiley-VCH, New York, 2003.
|
|
(b) Hua, X.; Liu, N.; Fan, Z.; Zong, G.; Ma, Y.; Lei, K.; Yin, H.; Wang, G. Chin. J. Org. Chem. 2019, 39,2581. (in Chinese)
|
|
|
( 华学文, 刘南南, 范志金, 宗广宁, 马翼, 雷康, 殷昊, 王桂清, 有机化学, 2019, 39,2581.)
|
|
|
(c) Zhong, L.; Jiang, T.; Zhang, F.; Fu, Q.; Liu, X.; Xu, T.; Ding, C.; Chen, J.; Yuan, J.; Tan, C. Chin. J. Org. Chem. 2019, 39,2655. (in Chinese)
|
|
|
( 钟良坤, 江涛, 张帆, 付庆, 刘幸海, 许天明, 丁成荣, 陈杰, 袁静, 谭成侠, 有机化学, 2019, 39,2655.)
|
|
| [2] |
For reviews, see: (a) Allen, C. L; Williams, J. M. J. Chem. Soc. Rev. 2011, 40,3405.
pmid: 27934465 |
|
(b) Pattabiraman, V.R.; Bode, J.W. Nature 2011, 480,471.
pmid: 27934465 |
|
|
(c) Lundberg, H.; Tinnis, F.; Selander, N.; Adolfsson, H. Chem. Soc. Rev. 2014, 43,2714.
pmid: 27934465 |
|
|
(d) Wan, J.-P.; Jing, Y. Beilstein J. Org. Chem. 2015, 11,2209.
pmid: 27934465 |
|
|
(e) de Figueiredo, R.M.; Suppo, J.-S.; Campagne, J.-M. Chem. Rev. 2016, 116,12029.
doi: 10.1021/acs.chemrev.6b00237 pmid: 27934465 |
|
|
(f) Ojeda-Porras, A.; Gamba-Sánchez, D. J. Org. Chem. 2016, 81,11548.
pmid: 27934465 |
|
|
For recent examples on the synthesis of amide, see: (g) Wang, Z.; Yang, L.; Liu, H.; Bao, W.; Tan, Y.; Wang, M.; Tang, Z.; He, W. Chin. J. Org. Chem. 2018, 38,2639. (in Chinese)
pmid: 27934465 |
|
|
( 王峥, 杨柳, 刘慧兰, 谭英芝, 包文虎, 汪明, 唐子龙, 何卫民, 有机化学, 2018, 38,2639.)
pmid: 27934465 |
|
|
(h) Xie, L.Y.; Peng, S.; Liu, F.; Yi, J.Y.; Wang, M.; Tang, Z.L.; Xu, X.H.; He, W.M. Adv. Synth. Catal. 2018, 360,4259.
pmid: 27934465 |
|
|
(i) Dissanayake, D. M., M.M.; Melville, A.D.; Vannucci, A.K. Green Chem. 2019, 21,3165.
pmid: 27934465 |
|
|
(j) Tu, Z.; Du, Y.; Cao, X.; Liu, Y. Adv. Synth. Catal. 2019, 361,4989.
pmid: 27934465 |
|
|
(k) Xie, L.-Y.; Hu, J.-L.; Song, Y.-X.; Jia, G.-K.; Lin, Y.-W.; He, J.-Y.; Cao, Z.; He, W.-M. ACS Sustainable Chem. Eng. 2019, 7,19993.
pmid: 27934465 |
|
|
(l) Yue, H.; Bao, P.; Wang, L.; Lü, X.; Yang, D.; Wang, H.; Wei, W. Chin. J. Org. Chem. 2019, 39,463. (in Chinese)
pmid: 27934465 |
|
|
( 岳会兰, 鲍鹏丽, 王雷雷, 吕晓霞, 杨道山, 王桦, 魏伟, 有机化学, 2019, 39,463.)
pmid: 27934465 |
|
|
(m) Sheng, R.; Li, P.; Zhou, Z.; Hu, G.; Zhang, X. Chin. J. Org. Chem. 2020, 40,462. (in Chinese)
pmid: 27934465 |
|
|
( 圣戎, 李萍, 周志强, 胡贵文, 张小祥, 有机化学, 2020, 40,462.)
pmid: 27934465 |
|
| [3] |
(a) Lanigan, R.M.; Sheppard, T.D. Eur. J. Org. Chem. 2013,7453.
pmid: 23473076 |
|
(b) Zheng, J.-F.; Jin, L.-R.; Huang, P.-Q. Org. Lett. 2004, 6,1139.
doi: 10.1021/ol049887k pmid: 23473076 |
|
|
(c) Tyrrell, E.; Brawn, P.; Carew, M.; Greenwood, I. Tetrahedron Lett. 2011, 52,369.
pmid: 23473076 |
|
|
(d) Allen, C.L.; Atkinson, B.N.; Williams, J.M. J. Angew. Chem. Int. Ed. 2012, 51,1383.
pmid: 23473076 |
|
|
(e) Rao, S.N.; Mohan, D.C.; Adimurthy, S. Org. Lett. 2013, 15,1496.
doi: 10.1021/ol4002625 pmid: 23473076 |
|
| [4] |
(a) Eldred, S.E.; Stone, D.A.; Gellman, S.H.; Stahl, S.S. J. Am. Chem. Soc. 2003, 125,3422.
doi: 10.1021/ja028242h pmid: 12643691 |
|
(b) Hoerter, J.M.; Otte, K.M.; Gellman, S.H.; Cui, Q.; Stahl, S.S. J. Am. Chem. Soc. 2008, 130,647.
pmid: 12643691 |
|
| [5] |
Bon, E.; Bigg, D.C. H.; Bertrand, G. J. Org. Chem. 1994, 59,4035.
|
| [6] |
(a) Gotor, V.; Brieva, R.; González, C.; Rebolledo, F. Tetrahedron 1991, 47,9207.
|
|
(b) Sergeeva, M.V.; Mozhaev, V.V.; Rich, J.O.; Khmelnitsky, Y.L. Biotechnol. Lett. 2000, 22,1419.
|
|
| [7] |
(a) Hie, L.; Fine Nathel, N.F.; Shah, T.K.; Baker, E.L.; Hong, X.; Yang, Y.-F.; Liu, P.; Houk, K.N.; Garg, N.K. Nature 2015, 524,79.
pmid: 26673267 |
|
(b) Weires, N.A.; Baker, E.L.; Garg, N.K. Nat. Chem. 2016, 8,75.
doi: 10.1038/nchem.2388 pmid: 26673267 |
|
| [8] |
(a) Baker, E.L.; Yamano, M.M.; Zhou, Y.; Anthony, S.M.; Garg, N.K. Nat. Commun. 2016, 7,11554.
doi: 10.1038/ncomms11554 pmid: 29163929 |
|
(b) Dander, J.E.; Baker, E.L.; Garg, N.K. Chem. Sci. 2017, 8,6433.
doi: 10.1039/c7sc01980g pmid: 29163929 |
|
| [9] |
(a) Meng, G.; Lei, P.; Szostak, M. Org. Lett. 2017, 19,2158.
pmid: 28397498 |
|
(b) Shi, S.; Szostak, M. Chem. Commun. 2017, 53,10584.
pmid: 28397498 |
|
| [10] |
Li, G.; Szostak, M. Chem. Rec. 2020, 20,649.
doi: 10.1002/tcr.201900072 pmid: 31833633 |
| [11] |
(a) Liu, Y.; Shi, S.; Achtenhagen, M.; Liu, R.; Szostak, M. Org. Lett. 2017, 19,1614.
doi: 10.1021/acs.orglett.7b00429 pmid: 31203613 |
|
(b) Li, G.; Szostak, M. Nat. Commun. 2018, 9,4165.
pmid: 31203613 |
|
|
(c) Liu, Y.; Achtenhagen, M.; Liu, R.; Szostak, M. Org. Biomol. Chem. 2018, 16,1322.
pmid: 31203613 |
|
|
(d) Li, G.; Ji, C.-L.; Hong, X.; Szostak, M. J. Am. Chem. Soc. 2019, 141,11161.
doi: 10.1021/jacs.9b04136 pmid: 31203613 |
|
| [12] |
Guo, W.; Huang, J.; Wu, H.; Liu, T.; Luo, Z.; Jian, J.; Zeng, Z. Org. Chem. Front. 2018, 5,2950.
|
| [13] |
Verho, O.; Pourghasemi Lati, M.; Oschmann, M. J. Org. Chem. 2018, 83,4464.
|
| [14] |
Rahman, M.M.; Li, G.; Szostak, M. J. Org. Chem. 2019, 84,12091.
pmid: 31430149 |
| [15] |
Ramkumar, R.; Chandrasekaran, S. Synthesis 2018, 51,921.
|
| [16] |
Other recent methods referring to the transamidation of amides (a) Cheung, C.W.; Ploeger, M. L.; Hu, X.; ACS Catal. 2017, 7,7092.
pmid: 32298591 |
|
(b) Cheung, C.W.; Ma, J.-A.; Hu, X. J. Am. Chem. Soc. 2018, 140,6789.
doi: 10.1021/jacs.8b03739 pmid: 32298591 |
|
|
(c) Mishra, A.; Singh, S.; Srivastava, V. Asian J. Org. Chem. 2018, 7,1600.
pmid: 32298591 |
|
|
(d) Ghosh, T.; Jana, S.; Dash, J. Org. Lett. 2019, 21,6690.
pmid: 32298591 |
|
|
(e) Sureshbabu, P.; Azeez, S.; Chaudhary, P.; Kandasamy, J. Org. Biomol. Chem. 2019, 17,845.
doi: 10.1039/c8ob03010c pmid: 32298591 |
|
|
(f) Chen, J.; Xia, Y.; Lee, S. Org. Lett. 2020, 22,3504.
doi: 10.1021/acs.orglett.0c00958 pmid: 32298591 |
|
| [17] |
Ye, D.; Liu, Z.; Chen, H.; Sessler, J.L.; Lei, C. Org. Lett. 2019, 21,6888.
|
| [18] |
(a) Galli, C. Org. Prep. Proced. Int. 1992, 24,285.
pmid: 11856006 |
|
(b) Salvatore, R.N.; Nagle, A.S.; Jung, K.W. J. Org. Chem. 2002, 67,674.
pmid: 11856006 |
|
| [19] |
(a) Meng, G.; Szostak, M. Angew. Chem., nt. Ed. 2015, 54,14518.
pmid: 27304392 |
|
(b) Meng, G.; Szostak, M. Org. Lett. 2015, 17,4364.
doi: 10.1021/acs.orglett.5b02209 pmid: 27304392 |
|
|
(c) Shi, S.; Meng, G.; Szostak, M. Angew. Chem., nt. Ed. 2016, 55,6959.
pmid: 27304392 |
|
|
(d) Shi, S.; Szostak, M. Chem.-Eur. J. 2016, 22,10420.
pmid: 27304392 |
|
| [20] |
Graton, J.; Berthelot, M.; Laurence, C. J. Chem. Soc., erkin Trans. 2 2001,2130.
|
| [21] |
The pKa of protonated N-methyl-4-methoxybenzylamine was 9.97 from website: http://ibond.nankai.edu.cn/pka/.
|
| [22] |
Bonnet, U. CNS Drug Rev. 2002, 8,283.
pmid: 12353059 |
| [23] |
Dworkin, R.H.; Kirkpatrick, P. Nat. Rev. Drug Discovery 2005, 4,455.
doi: 10.1038/nrd1756 pmid: 15959952 |
| [24] |
Sheraz, M.A.; Ahsan, S.F.; Khan, M.F.; Ahmed, S.; Ahmad, I. J. Pharm. 2016, 2016,8961621.
|
| [25] |
Bryans, J.S.; Williams, S.C.; Blakemore, D.C. EP 1178034, 2001.
|
| [26] |
(a) Yamada, S.; Hongo, C.; Yoshioka, R.; Chibata, I. J. Org. Chem. 1983, 48,843.
|
|
(b) Harry, L.G.; Pugnière, M.; Castro, B.; Previero, A. Int. J. Peptide Prorein Res. 1993, 41,323.
|
|
|
(c) Ramachandran, U.; Kumar, S.; Chawla, H.P. S. Org. Prep. Proced. Int. 2003, 35,616.
|
|
|
(d) Wang, S.M.; Zhao, C.; Zhang, X.; Qin, H.L. Org. Biomol. Chem. 2019, 17,4087.
|
|
| [27] |
(a) Cheung, C.W.; Ploeger, M.L.; Hu, X. Nat. Commun. 2017, 8,14878.
doi: 10.1038/ncomms14878 pmid: 28345585 |
|
(b) Halima, T.B.; Masson-Makdissi, J.; Newman, S.G. Angew. Chem. Int. Ed. 2018, 57,12925.
pmid: 28345585 |
|
| [28] |
(a) D, H.A.; Hang, Y. EP 20050713917, 2005.
pmid: 16634594 |
|
(b) Abergel, R.J.; Raymond, K.N. Inorg. Chem. 2006, 45,3622.
pmid: 16634594 |
|
| [29] |
Kanzian, T.; Nigst, T.A.; Maier, A.; Pichl, S.; Mayr, H. Eur. J. Org. Chem. 2009,6379.
|
| [30] |
Fox, J.M.; Dmitrenko, O.; Liao, L.-a.; Bach, R.D. J. Org. Chem. 2004, 69,7317.
doi: 10.1021/jo049494z pmid: 15471486 |
| [31] |
Adachi, S.; Kumagai, N.; Shibasaki, M. Tetrahedron Lett. 2018, 59,1147.
|
| [32] |
Kumar, V.; Connon, S.J. Chem. Commun. 2017, 53,10212.
|
| [33] |
Wang, X.-F.; Yu, S.-S.; Wang, C.; Xue, D.; Xiao, J. Org. Biomol. Chem. 2016, 14,7028.
doi: 10.1039/c6ob00736h pmid: 27363514 |
| [34] |
Chardon, A.; Mohy El Dine, T.; Legay, R.; De Paolis, M.; Rouden, J.; Blanchet, J. Chem.-Eur. J. 2017, 23,2005.
pmid: 27930832 |
| [35] |
Ning, X.-Q.; Lou, S.-J.; Mao, Y.-J.; Xu, Z.-Y.; Xu, D.-Q. Org. Lett. 2018, 20,2445.
doi: 10.1021/acs.orglett.8b00793 pmid: 29634276 |
| [36] |
Zhang, B.; Feng, P.; Cui, Y.; Jiao, N. Chem. Commun. 2012, 48,7280.
|
| [37] |
Chaudhari, M.B.; Bisht, G.S.; Kumari, P.; Gnanaprakasam, B. Org. Biomol. Chem. 2016, 14,9215.
|
| [38] |
Mondal, A.; Subaramanian, M.; Nandakumar, A.; Balaraman, E. Org. Lett. 2018, 20,3381.
doi: 10.1021/acs.orglett.8b01305 pmid: 29791162 |
| [39] |
Qian, C.; Zhang, X.; Zhang, Y.; Shen, Q. J. Organomet. Chem. 2010, 695,747.
|
| [40] |
Zhu, M.; Fujita, K.-i.; Yamaguchi, R. J. Org. Chem. 2012, 77,9102.
doi: 10.1021/jo301553v pmid: 23006061 |
| [41] |
Sawant, D.N.; Bagal, D.B.; Ogawa, S.; Selvam, K.; Saito, S. Org. Lett. 2018, 20,4397.
pmid: 30020789 |
| [42] |
Zhu, J.; Zhang, Y.; Shi, F.; Deng, Y. Tetrahedron Lett. 2012, 53,3178.
|
| [43] |
Soulard, V.; Villa, G.; Vollmar, D.P.; Renaud, P. J. Am. Chem. Soc. 2018, 140,155.
doi: 10.1021/jacs.7b12105 pmid: 29240406 |
| [44] |
Zhao, Q.; Li, H.; Wang, L. Org. Biomol. Chem. 2013, 11,6772.
pmid: 23999992 |
| [45] |
Hamada, S.; Sugimoto, K.; Iida, M.; Furuta, T. Tetrahedron Lett. 2019, 60,151277.
|
| [46] |
Rossi, S.A.; Shimkin, K.W.; Xu, Q.; Mori-Quiroz, L.M.; Watson, D.A. Org. Lett. 2013, 15,2314.
doi: 10.1021/ol401004r pmid: 23611591 |
| [47] |
Yuan, Y.-C.; Kamaraj, R.; Bruneau, C.; Labasque, T.; Roisnel, T.; Gramage-Doria, R. Org. Lett. 2017, 19,6404.
pmid: 29152976 |
| [48] |
Chernykh, A.V.; Melnykov, K.P.; Tolmacheva, N.A.; Kondratov, I.S.; Radchenko, D.S.; Daniliuc, C.G.; Volochnyuk, D.M.; Ryabukhin, S.V.; Kuchkovska, Y.O.; Grygorenko, O.O. J. Org. Chem. 2019, 84,8487.
doi: 10.1021/acs.joc.9b00719 pmid: 30990713 |
| [49] |
Tran, B.L.; Li, B.; Driess, M.; Hartwig, J.F. J. Am. Chem. Soc. 2014, 136,2555.
pmid: 24405209 |
| [50] |
Wang, Y.; Hu, X.; Morales-Rivera, C.A.; Li, G.-X.; Huang, X.; He, G.; Liu, P.; Chen, G. J. Am. Chem. Soc. 2018, 140,9678.
doi: 10.1021/jacs.8b05753 pmid: 29983059 |
| [1] | Jie Liu, Feng Han, Shuangyan Li, Tianyu Chen, Jianhui Chen, Qing Xu. Transition Metal-Free Selective Aerobic Olefination of Methyl N-Heteroarenes with Alcohols [J]. Chinese Journal of Organic Chemistry, 2024, 44(2): 573-583. |
| [2] | Zhongrong Xu, Jieping Wan, Yunyun Liu. Transition Metal-Free C—H Thiocyanation and Selenocyanation Based on Thermochemical, Photocatalytic and Electrochemical Process [J]. Chinese Journal of Organic Chemistry, 2023, 43(7): 2425-2446. |
| [3] | Jiao Qin, Jie Chen, Yan Su. Synthesis of 2,2,6,6-Tetramethylpiperidin-1-yl-2-(2-cyanophenyl)-acetate by Transition Metal-Free Radical Cleavage Reaction from α-Bromoindanone [J]. Chinese Journal of Organic Chemistry, 2023, 43(6): 2171-2177. |
| [4] | Xiantao Ma, Xiaoyu Yan, Yingying Zhu, Shuanglin Niu, Yuxuan Wang, Chao Yuan. Water-Promoted Green Synthesis of Heteroaryl Thioether [J]. Chinese Journal of Organic Chemistry, 2023, 43(6): 2136-2142. |
| [5] | Jing Sun, Mengmeng Zhang, Xiaolong Guo, Qi Wang, Luyao Wang. Synthesis of Diaryl Selenium Compounds without Transition-Metal Catalyst [J]. Chinese Journal of Organic Chemistry, 2023, 43(12): 4251-4260. |
| [6] | Haojie Ma, Fengyuan Zhou, Fanwen Su, Bo Han, Ran Li, Yuqi Zhang, Jijiang Wang. Iodine-Promoted Transamidation of N,N-Dimethylacetamide (DMA) with Amines [J]. Chinese Journal of Organic Chemistry, 2023, 43(11): 3960-3965. |
| [7] | Tianyu Chen, Feng Han, Shuangyan Li, Jianping Liu, Jianhui Chen, Qing Xu. Transition Metal-Free Selective Aerobic C-Alkylation of Methyl N-Heteroarenes with Alcohols [J]. Chinese Journal of Organic Chemistry, 2022, 42(9): 2914-2924. |
| [8] | Qun Han, Kun Xu, Faning Tian, Shengyang Huang, Chengchu Zeng. A Practical Transamidation Strategy for the N-Deacylation of Amides [J]. Chinese Journal of Organic Chemistry, 2022, 42(4): 1123-1128. |
| [9] | Juyan Liu, Congying Zhao. Lactic Acid-Catalyzed Transamidation Reactions of Carboxamides with Amines [J]. Chinese Journal of Organic Chemistry, 2021, 41(6): 2310-2318. |
| [10] | Qi Wang, Boran Zhu, Guang Yang, Xiantao Ma, Qing Xu. Selective Synthesis of Unsymmetrical N-Heteroaryl Thioethers byBase-Free Direct Multi-Component Reaction [J]. Chinese Journal of Organic Chemistry, 2021, 41(3): 1193-1199. |
| [11] | Li Liu, Hong Xiao, Fuhong Xiao, Yanjun Xie, Huawen Huang, Guojun Deng. Synthesis of β-Ketosulfone from Sodium Sulfinate and Aryl Ethyl Ketone/Indanone [J]. Chinese Journal of Organic Chemistry, 2021, 41(12): 4749-4757. |
| [12] | Baoli Zhao, Liangfeng Yang, Kai Cheng, Liyun Zhou, Jie-Ping Wan. Visible Light Induced Oxidation of α-Diazo Esters for the Transition Metal-Free Synthesis of α-Keto Esters [J]. Chinese Journal of Organic Chemistry, 2021, 41(12): 4732-4737. |
| [13] | Kong Yaolei, Sun Xiaotong, Weng Jianquan. Selectfluor as “Fluorine-Free” Functional Reagent Applied to Organic Synthesis under Transition Metal-Free Conditions [J]. Chinese Journal of Organic Chemistry, 2020, 40(9): 2641-2657. |
| [14] | Xu Xinming, Yang Hanlin, Li Wenzhong. Transition Metal-Free Direct C-H Bond Sulfenylation of Alkenes and Arenes [J]. Chinese Journal of Organic Chemistry, 2020, 40(7): 1912-1925. |
| [15] | Zhang Lei, Yuan Sitian, Wang Peng, Liu Jinbiao. Recent Advances in Cyclization Reaction of Alkynes under Transition Metal-Free Conditions [J]. Chinese Journal of Organic Chemistry, 2020, 40(6): 1529-1539. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||