Chinese Journal of Organic Chemistry ›› 2021, Vol. 41 ›› Issue (6): 2343-2353.DOI: 10.6023/cjoc202101049 Previous Articles Next Articles
ARTICLES
收稿日期:2021-01-28
修回日期:2021-02-24
发布日期:2021-03-22
通讯作者:
王明安
基金资助:
Weiwei Wang( ), Yu Zhao, Xinlei Liu, Jiazhen Jiang, Ming'an Wang
), Yu Zhao, Xinlei Liu, Jiazhen Jiang, Ming'an Wang
Received:2021-01-28
Revised:2021-02-24
Published:2021-03-22
Contact:
Ming'an Wang
Supported by:Share
Weiwei Wang, Yu Zhao, Xinlei Liu, Jiazhen Jiang, Ming'an Wang. Synthesis and Antifungal Activity of 3-Aryl-7-methyl- 7-hydroxy-2-octen-6-olide[J]. Chinese Journal of Organic Chemistry, 2021, 41(6): 2343-2353.
| Entry | Ar | Olefin acid | Lactone | Yield/% |
|---|---|---|---|---|
| 1 | C6H5 | 1-13a | 5a | 80 |
| 2 | 2-ClC6H4 | 1-13b | 5b | 91 |
| 3 | 3-ClC6H4 | 1-13c | 5c | 84 |
| 4 | 4-ClC6H4 | 1-13d | 5d | 69 |
| 5 | 4-CH3OC6H4 | 1-13e | 5e | 61 |
| 6 | 4-CH3C6H4 | 1-13f | 5f | 75 |
| 7 | 4-FC6H4 | 1-13g | 5g | 70 |
| 8 | 4-CF3C6H4 | 1-13h | 5h | 81 |
| 9 | 4-PhC6H4 | 1-13i | 5i | 82 |
| 10 | 1-C10H7 | 1-13j | 5j | 73 |
| 11 | 2-C10H7 | 1-13k | 5k | 84 |
| 12 | 4-CH3COC6H4 | 1-13l | 5l | 68 |
| 13 | 4-CH3O2CC6H4 | 1-13m | 5m | 78 |
| 14 | 4-(CH3)3CC6H4 | 1-13n | 5n | 90 |
| 15 | 4-CNC6H4 | 1-13o | 5o | 65 |
| 16 | 2-CH3OC6H4 | 1-13p | 5p | 88 |
| 17 | 2-CH3C6H4 | 1-13q | 5q | 81 |
| 18 | 2,4-(CH3)2C6H4 | 1-13r | 5r | 79 |
| 19 | 3,5-(CH3)2C6H4 | 1-13s | 5s | 85 |
| Entry | Ar | Olefin acid | Lactone | Yield/% |
|---|---|---|---|---|
| 1 | C6H5 | 1-13a | 5a | 80 |
| 2 | 2-ClC6H4 | 1-13b | 5b | 91 |
| 3 | 3-ClC6H4 | 1-13c | 5c | 84 |
| 4 | 4-ClC6H4 | 1-13d | 5d | 69 |
| 5 | 4-CH3OC6H4 | 1-13e | 5e | 61 |
| 6 | 4-CH3C6H4 | 1-13f | 5f | 75 |
| 7 | 4-FC6H4 | 1-13g | 5g | 70 |
| 8 | 4-CF3C6H4 | 1-13h | 5h | 81 |
| 9 | 4-PhC6H4 | 1-13i | 5i | 82 |
| 10 | 1-C10H7 | 1-13j | 5j | 73 |
| 11 | 2-C10H7 | 1-13k | 5k | 84 |
| 12 | 4-CH3COC6H4 | 1-13l | 5l | 68 |
| 13 | 4-CH3O2CC6H4 | 1-13m | 5m | 78 |
| 14 | 4-(CH3)3CC6H4 | 1-13n | 5n | 90 |
| 15 | 4-CNC6H4 | 1-13o | 5o | 65 |
| 16 | 2-CH3OC6H4 | 1-13p | 5p | 88 |
| 17 | 2-CH3C6H4 | 1-13q | 5q | 81 |
| 18 | 2,4-(CH3)2C6H4 | 1-13r | 5r | 79 |
| 19 | 3,5-(CH3)2C6H4 | 1-13s | 5s | 85 |
| Compd. | R. solani | A. solani | S. sclerotiorum | B. cinerea | F. graminearum | P. capsici |
|---|---|---|---|---|---|---|
| 5a | 10.7 | 3.7 | 5.1 | 0.5 | 19.4 | 18.6 |
| R-5a | 4.6 | 2.4 | 2.8 | 0.2 | 13.5 | 12.0 |
| S-5a | 15.5 | 9.8 | 10.4 | 1.4 | 26.2 | 25.7 |
| 5d | —a | — | 12.3 | — | — | — |
| 5i | 35.9 | — | 37.5 | 11.3 | — | 35.0 |
| R-5i | 12.6 | — | 13.3 | 9.7 | — | 17.9 |
| S-5i | 62.8 | — | 68.6 | 24.5 | — | 65.3 |
| 5j | — | — | 12.8 | — | — | — |
| 5k | 104.0 | — | 11.6 | 23.7 | — | 40.0 |
| R-5k | 64.5 | — | 4.8 | 8.6 | — | 22.7 |
| S-5k | 150.8 | — | 24.4 | 35.8 | — | 68.2 |
| 5n | 54.1 | — | 15.2 | — | — | 57.6 |
| R-5n | 38.2 | — | 8.3 | — | — | 22.1 |
| S-5n | 84.5 | — | 30.8 | — | — | 88.6 |
| Carbendazim | 0.05 | 0.05 | 7.9 | 528.2 | 0.07 | 77.8 |
| Compd. | R. solani | A. solani | S. sclerotiorum | B. cinerea | F. graminearum | P. capsici |
|---|---|---|---|---|---|---|
| 5a | 10.7 | 3.7 | 5.1 | 0.5 | 19.4 | 18.6 |
| R-5a | 4.6 | 2.4 | 2.8 | 0.2 | 13.5 | 12.0 |
| S-5a | 15.5 | 9.8 | 10.4 | 1.4 | 26.2 | 25.7 |
| 5d | —a | — | 12.3 | — | — | — |
| 5i | 35.9 | — | 37.5 | 11.3 | — | 35.0 |
| R-5i | 12.6 | — | 13.3 | 9.7 | — | 17.9 |
| S-5i | 62.8 | — | 68.6 | 24.5 | — | 65.3 |
| 5j | — | — | 12.8 | — | — | — |
| 5k | 104.0 | — | 11.6 | 23.7 | — | 40.0 |
| R-5k | 64.5 | — | 4.8 | 8.6 | — | 22.7 |
| S-5k | 150.8 | — | 24.4 | 35.8 | — | 68.2 |
| 5n | 54.1 | — | 15.2 | — | — | 57.6 |
| R-5n | 38.2 | — | 8.3 | — | — | 22.1 |
| S-5n | 84.5 | — | 30.8 | — | — | 88.6 |
| Carbendazim | 0.05 | 0.05 | 7.9 | 528.2 | 0.07 | 77.8 |

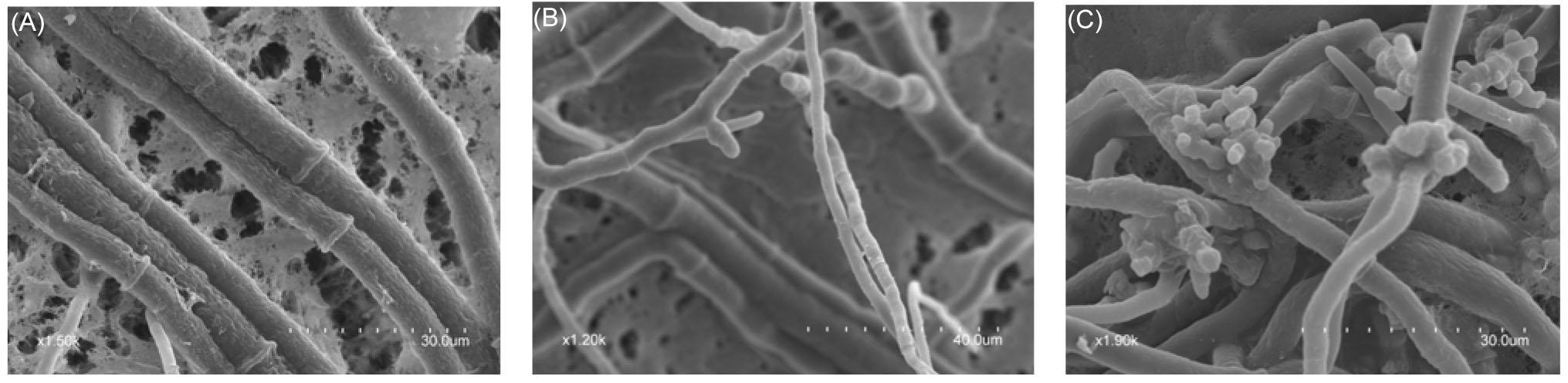

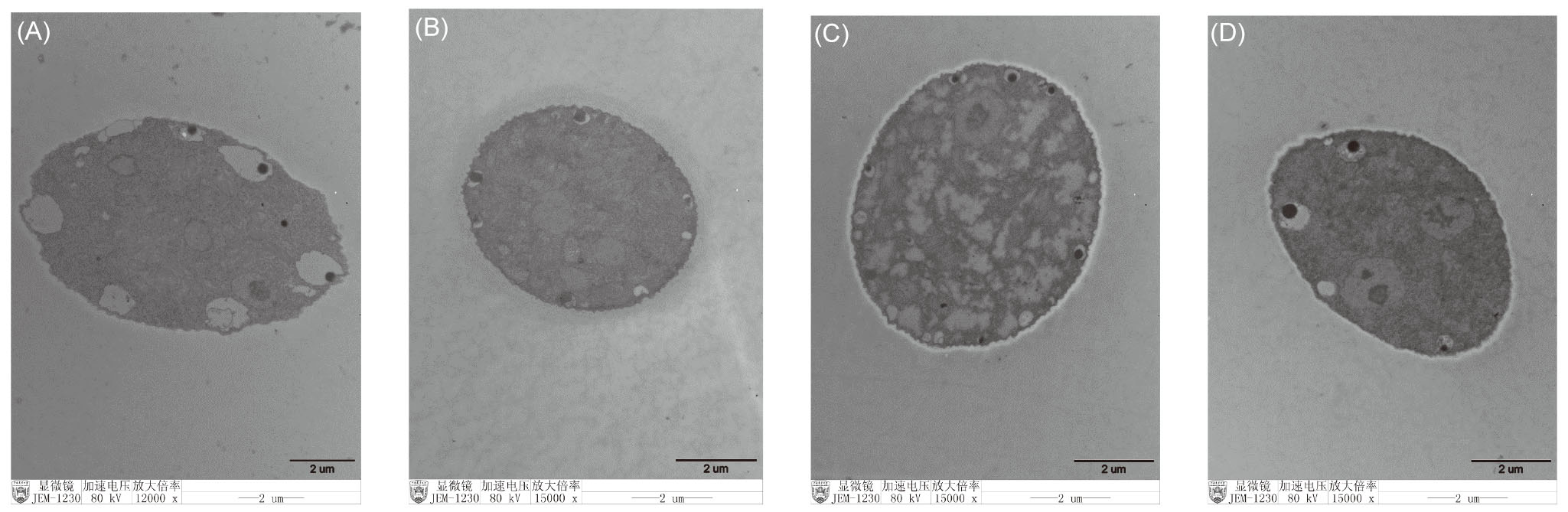
| [1] |
Gabriela, L.; Gallardo, N. I. P.; Cabrera, G. M. Phytochem. Lett. 2008, 1,155.
doi: 10.1016/j.phytol.2008.07.008 |
| [2] |
Yang, Y.; Jiang, J. Z.; Qimei, L. B.; Yan, X. J.; Zhao, J. X.; Yuan, H. Z.; Qin, Z. H.; Wang, M. A. Molecules 2010, 15,7075.
doi: 10.3390/molecules15107075 pmid: 20944522 |
| [3] |
Schulz, S.; Hotling, S. Nat. Prod. Rep. 2015, 32,1042.
doi: 10.1039/C5NP00006H |
| [4] |
Davies-Coleman, M. T.; Rivett, D. E. A. Prog. Chem. Org. Nat. Prod. 1989, 55,1.
|
| [5] |
Collett, L. A.; Davies-Coleman, M. T.; Rivett, D. E. A. Prog. Chem. Org. Nat. Prod. 1998, 75,182.
|
| [6] |
Pereda-Miranda, R.; Fragoso-Serrano, M.; Cerda-Garcia-Rojas, C. M. Tetrahedron 2001, 57,47.
doi: 10.1016/S0040-4020(00)00987-X |
| [7] |
Hoffmann, H. M. R.; Rabe, J. Angew. Chem., Int. Ed. 1985, 24,94.
doi: 10.1002/(ISSN)1521-3773 |
| [8] |
Marco, J. A.; Carda, M.; Murga, J.; Falomir, E. Tetrahedron 2007, 63,2929.
doi: 10.1016/j.tet.2006.12.047 |
| [9] |
Boucard, V.; Broustal, G.; Campagne, J. M. Eur. J. Org. Chem. 2007,225.
|
| [10] |
Gerard, R. Tetrahedron 1995, 51,2777.
doi: 10.1016/0040-4020(94)01064-7 |
| [11] |
Helena, M. C.; Fernanda, I. B.; Myrian, K. S.; Luiz, S. L. J. Quim. Nova 2008, 31,885.
doi: 10.1590/S0100-40422008000400029 |
| [12] |
Helena, M. C.; Fernanda, I. B.; Luiz, S. L. J. Synthesis 2007,3261.
|
| [13] |
Shiina, I. Chem. Rev. 2007, 107,239.
pmid: 17212476 |
| [14] |
Chang, H.-T.; Jayanth, T. T.; Wang, C.-C.; Cheng, C.-H. J. Am. Chem. Soc. 2007, 129,12032.
doi: 10.1021/ja073604c |
| [15] |
Ebine, M.; Suga, Y.; Fuwa, H.; Sasaki, M. Org. Biomol. Chem. 2010, 8,39.
doi: 10.1039/B919673K |
| [16] |
Emily, B. P.; Tendai, G.; Sonbinh, T. N. Org. Lett. 2008, 10,5613.
doi: 10.1021/ol8022227 |
| [17] |
van, As, B. A. C.; Chan, D.; Kivit, P. J. J.; Palmans, A. R. A.; Meijer, E. W. Tetrahedron: Asymmetry 2007, 18,787.
doi: 10.1016/j.tetasy.2007.03.016 |
| [18] |
Maxime, A. S.; Huub, K.; Anthony, L. S. Acta Cryst. 2008,E64, o607.
|
| [19] |
Fukuzawa, A.; Masamune, T. Tetrahedron Lett. 1981, 22, 4081.
doi: 10.1016/S0040-4039(01)82070-0 |
| [20] |
Cremer, D. J.; Pople, A. J. Am. Chem. Soc. 1975, 97,1354.
doi: 10.1021/ja00839a011 |
| [21] |
Wandjia, J.; Wansi, J. D.; Fuendjiep, V.; Dagne, E.; Mulholland, D. A.; Tillequin, F.; Fomum, Z. T.; Sondengam, B. L.; Nkeh, B. C.; Njamen, D. Phytochemistry 2000, 54,811.
pmid: 11014271 |
| [22] |
Nkeh, B. C.; Njamen, D.; Wandjia, J.; Fomum, Z. T.; Dongmo, A.; Nguelefack, T. B.; Wansi, J. D.; Kamanyi, A. Pharm. Biol. 2003, 41,26.
doi: 10.1076/phbi.41.1.26.14704 |
| [23] |
Jhulki, S.; Seth, S.; Mondal, M.; Moorthy, J. N. Tetrahedron 2014, 70,2286.
doi: 10.1016/j.tet.2014.01.034 |
| [24] |
Kadnikov, D. V.; Larock, R. C. Mendeleev Commun. 2007, 17,74.
doi: 10.1016/j.mencom.2007.03.006 |
| [25] |
Cheng, Y. A.; Chen, T.; Tan, C. K.; Heng, J. J.; Yeung, Y. Y. J. Am. Chem. Soc. 2012, 134,16492.
doi: 10.1021/ja307210n pmid: 22998611 |
| [26] |
Wang, L.; Ren, Z.-L.; Ding, M.-W. J. Org. Chem. 2015, 80,641.
doi: 10.1021/jo502275f |
| [27] |
Arcadi, A.; Fabrizi, G.; Goggiamani, A.; Marinelli, F. J. Org. Chem. 2015, 80,6986.
doi: 10.1021/acs.joc.5b00663 |
| [28] |
Matsuda, T.; Shigeno, M.; Murakami, M. Org. Lett. 2008, 10,5219.
doi: 10.1021/ol802218a |
| [29] |
Zarrabi, S.; Mahmoodi, N. O.; Tabatabaeian, K.; Zanjanchi, M. A. Chin. Chem. Lett. 2009, 20,1400.
doi: 10.1016/j.cclet.2009.07.007 |
| [30] |
Dong, H. B.; Yang, M. Y.; Jiang, J. Z.; Wang, M. A. J. Asian Nat. Prod. Res. 2013, 15,880.
doi: 10.1080/10286020.2013.803474 |
| [31] |
Dong, H. B.; Yang, M. Y.; Tang, B.; Wang, M. A. Chin. J. Org. Chem. 2014, 34,2350(in Chinese).
doi: 10.6023/cjoc201406005 |
|
( 董宏波, 杨明艳, 汤博, 王明安, 有机化学, 2014, 34,2350.)
doi: 10.6023/cjoc201406005 |
|
| [32] |
Zhao, J.; Dong, H. B.; Yang, M. Y.; Du, J.; Jiang, J. Z.; Wang, M. A. J. Asian Nat. Prod. Res. 2014, 16,312.
doi: 10.1080/10286020.2013.879121 pmid: 24456253 |
| [33] |
Dong, H. B.; Wang, W. W.; Zhao, Y.; Liu, X. L.; Wang, M. A. Chin. J. Org. Chem. 2021, 41,1646(in Chinese).
doi: 10.6023/cjoc202010011 |
|
( 董宏波, 王卫伟, 赵宇, 刘鑫磊, 王明安, 有机化学, 2021, 41,1646.)
doi: 10.6023/cjoc202010011 |
|
| [34] |
Yonova, I. M.; Johnson, A. G.; Osborne, C. A.; Moore, C. E.; Morrissette, N. S.; Jorvo, E. R. Angew. Chem., Int. Ed. 2014, 53,2422.
doi: 10.1002/anie.201308666 |
| [35] |
Touchard, F. P. Tetrahedron Lett. 2004, 45,5519.
doi: 10.1016/j.tetlet.2004.05.026 |
| [36] |
Wang, W. W.; Zhao, Y.; Liu, X. L.; Geng, R.; Wang, M. A. Chin. J. Org. Chem. 2019, 39,1129(in Chinese).
|
|
( 王卫伟, 赵宇, 刘鑫磊, 耿瑞, 王明安, 有机化学, 2019, 39,1129.)
doi: 10.6023/cjoc201809018 |
|
| [37] |
Tang, B.; Guan, A. Y.; Zhao, Y.; Jiang, J. Z.; Wang, M. A.; Zhou, L. G. Chin. J. Chem. 2017, 35,1133.
doi: 10.1002/cjoc.v35.7 |
| [38] |
Zhao, Y.; Tang, B.; Guan, A. Y.; Wang, W. W.; Zhang, Z. H.; Wang, M. A. Synthesis 2017, 49,4663.
doi: 10.1055/s-0036-1588463 |
| [39] |
Geng, R.; Zhao, Y.; Li, Y. H.; Liu, X. L.; Wang, M. A. Chin. J. Org. Chem. 2019, 39,3574(in Chinese).
doi: 10.6023/cjoc201906017 |
|
( 耿瑞, 赵宇, 李益豪, 刘鑫磊, 王明安, 有机化学, 2019, 39,3574.)
doi: 10.6023/cjoc201906017 |
|
| [40] |
Schwartz, B. D.; Tilly, D. P.; Heim, R.; Wiedemann, S.; Williams, C. M.; Bernhardt, P. V. Eur. J. Org. Chem. 2006,3181.
|
| [41] |
Anastasia, L.; Dumond, Y. R.; Negishi, E. Eur. J. Org. Chem. 2001,3039.
|
| [42] |
Derrick, L. J.; Clive, V. F.; Pierre, L. B. J. Org. Chem. 1982, 47,2572.
doi: 10.1021/jo00134a013 |
| [1] | Feng Wang, Yu Chen, Hongyan Pei, Jing Zhang, Lixin Zhang. Design, Synthesis and Antifungal Activities of Novel 1,2,4-Oxadiazole Derivatives Containing Piperidine [J]. Chinese Journal of Organic Chemistry, 2023, 43(8): 2826-2836. |
| [2] | Meng Li, Dongguo Xia, Yunxiao Wang, Xiang Cheng, Jiexiu Gong, Yao Chen, Xianhai Lü. Design, Synthesis and Antifungal Bioactivity Evaluation of Thiazole Benzoate Derivatives [J]. Chinese Journal of Organic Chemistry, 2023, 43(2): 686-696. |
| [3] | Wei Chen, Simin Lei, Yuxin Lan, Haojian Xu, Pingbin Yu, Rui Zhang, Run Wu, Yang Chen. Design, Synthesis and Antifungal Activities of Novel Quinazolinone Derivatives [J]. Chinese Journal of Organic Chemistry, 2022, 42(7): 2164-2171. |
| [4] | Xiu Wang, Wengui Duan, Guishan Lin, Baoyu Li, Wenjing Zhang, Fuhou Lei. Synthesis, Antifungal Activity, Three-Dimensional Quantitative Structure-Activity Relationship and Molecular Docking Study of 4-Acyl-3-amino-1,2,4-triazole-thioether Derivatives Containing Natural Pinene Structure [J]. Chinese Journal of Organic Chemistry, 2022, 42(3): 871-883. |
| [5] | Wei Wang, Furan Wu, Yidan Ma, Dan Xu, Gong Xu. Study on Synthesis and Antifungal Activity of Novel Benzamides Containing Substituted Pyrazole Unit [J]. Chinese Journal of Organic Chemistry, 2022, 42(2): 607-618. |
| [6] | Yucheng Cui, Meihua Chen, Guishan Lin, Wengui Duan, Qingmin Li, Renxuan Zou, Bo Cen. Synthesis, Antifungal Activity and Molecular Docking Study of 1,3,4-Thiadiazole-Urea Compounds Containing gem-Dimethylcyclopropane Ring Structure [J]. Chinese Journal of Organic Chemistry, 2022, 42(11): 3784-3797. |
| [7] | Weiwei Wang, Yihao Li, Xinlei Liu, Yu Zhao, Mingan Wang. Synthesis and Fungicidal Activity of Novel 3,7-Dimethylocta-2,6-dienamides and 3,7-Dimethyl-6,7-dihydroxyoct-2-enamides [J]. Chinese Journal of Organic Chemistry, 2021, 41(9): 3717-3725. |
| [8] | Hongbo Dong, Weiwei Wang, Yu Zhao, Xinlei Liu, Ming'an Wang. Synthesis and Antifungal Activity of 3,7-Dimethyl-7-hydroxy-2-octen-6-olide Analogues [J]. Chinese Journal of Organic Chemistry, 2021, 41(4): 1646-1657. |
| [9] | Xibin Wu, Yinfeng Tan, Jiling Yi, Xinming Song, Jingyu Yang, Xueming Zhou, Guangying Chen. Study on Bioactive Secondary Metabolites from Penicillium herquei JX4 [J]. Chinese Journal of Organic Chemistry, 2021, 41(3): 1251-1254. |
| [10] | Li Anbang, Li Zhongshan, Zhao Yang, Yao Tingting, Cheng Jingli, Zhao Jinhao. Design, Synthesis and Antifungal Activity of Novel Pyrazole-Thiophene Carboxamide Derivatives [J]. Chinese Journal of Organic Chemistry, 2020, 40(9): 2836-2844. |
| [11] | Jiang Zhenhua, Cheng Yi'nan, Shen Guofu, Zhang Mengmeng, Su Ziyang, Sun Liansheng, Li Honglian. Synthesis of 1,2,4-Triazole Benzamide Derivatives and Fungicidal Activity [J]. Chinese Journal of Organic Chemistry, 2020, 40(8): 2575-2582. |
| [12] | Xiao Tingting, Cheng Wei, Qian Weifeng, Zhang Tingting, Lu Tong, Gao Yang, Tang Xiaorong. Synthesis of Chalcone Derivatives and Studies on Their Inhibitory Activity and Molecular Docking [J]. Chinese Journal of Organic Chemistry, 2020, 40(6): 1704-1715. |
| [13] | Wang Ranran, Wang Yan, Bian Yanqing, Zhang Ping. Synthesis and Antifungal Activity of 1,5-Benzothiazepines Containing 1,2,3-Triazole [J]. Chinese Journal of Organic Chemistry, 2020, 40(2): 398-407. |
| [14] | Yan Yingkun, Cheng Wei, Xiao Tingting, Zhang Guilan, Zhang Tingting, Lu Tong, Tang Xiaorong. Discovery of Novel 2,4,6-Trisubstituted Pyrimidine Derivatives as Succinate Dehydrogenase Inhibitors [J]. Chinese Journal of Organic Chemistry, 2020, 40(12): 4237-4248. |
| [15] | Chen Wei, Zuo Huailong, Li Yuxin, Liu Jiang, Zhou Xianli. Design, Synthesis and Structure-Activity Relationships of Plant-Based 2-Aryl-3,4-dihydroisoquinolin-2-iums as Potential Antifungal Agents [J]. Chin. J. Org. Chem., 2019, 39(8): 2317-2322. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||